Phosphate ion (PO43-) testing in Qualitative Analysis | Phosphoric acids, compounds
There are tests to identify phosphate ion in qualitative analysis. Some metal phosphates are soluble in water and some are not. Physical characteristics such as precipitating, colour changes, vapor emissions and etc. are observed when PO43- ion is tested. The main source of phosphorous is apatite. Additionally, Phosphoric acid (H3PO4) is a weak acid. In many occasions phosphate ion give coloured precipitates. Phosphate ions are very important in fertilizing industry. In this tutorial, we will study how to identify phosphate ion from other anions.
Content
- Structure of phosphate ion (PO43-)
- Identify phosphate ion - Qualitative analysis
- Test for phosphate ion - Concentrated nitric acid and ammonium molybdate
- Test for phosphate ion - Silver nitrate and phosphate ion solution
- Test for phosphate ion with Barium chloride
- Barium chloride (BaCl2) and Sodium biphosphate (Na2HPO4) reaction
- Testing of phosphate ion with Ferric chloride
- Testing of phosphate ion With Magnesia mixture
- Fertilizer Production and phosphate ion
- Oxyacids of phosphorus
- Occurrence of phosphate ion
Structure of phosphate ion (PO43-)
According to the lewis structure of phosphate ion, there is only one double between center phosphorus atom and one oxygen atom. As well, there are three more three single bonds.
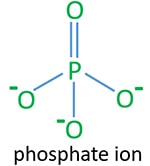
In this tutorial we first discuss about identifying phosphate ion (PO43-). Next we study some compounds and reactions of phosphorus and acids of phosphorus.
Identify phosphate ion - Qualitative analysis/
Test for phosphate ion - Concentrated nitric acid and ammonium molybdate
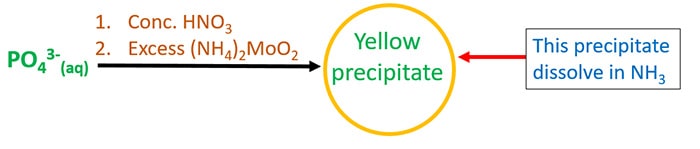
Add concentrated nitric acid (HNO3) to phosphate ion solution. Next, add excess ammonium molybdate (NH4)2MoO4. You can see a yellow precipitate forms in the solution. That yellow precipitate will dissolve in ammonia solution.
Test for phosphate ion - Silver nitrate and phosphate ion solution
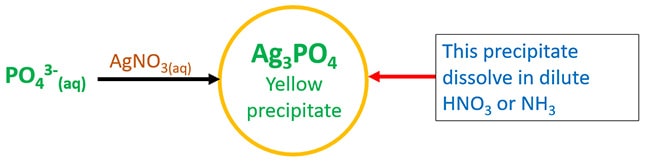
Silver nitrate and phosphate ion solution react and form silver phosphate ( Ag3PO4 ) which is a yellow precipitate. That precipitate dissolve in dilute nitric acid (HNO3) or in ammonia solutions.
Test for phosphate ion with Barium chloride
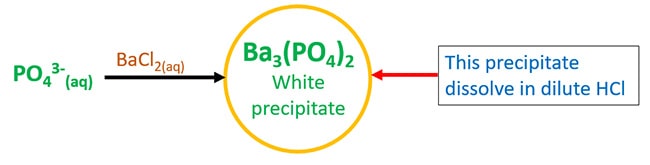
Barium chloride (BaCl2) aqueous solution and phosphate ion aqueous solution react and give Barium phosphate (Ba3(PO4)2), a white precipitate which dissolve in dilute hydrochloric acid (HCl).
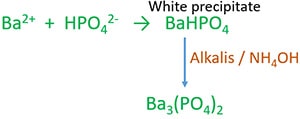
Barium chloride (BaCl2) and Sodium biphosphate (Na2HPO4) reaction
Barium chloride reacts with Sodium biphosphate solution to give white precipitate of BaHPO4. which is soluble in acids (except H2SO4).
In the presence of alkali or ammonium hydroxide, HPO43- ion is converted to phosphate ion (PO43-).
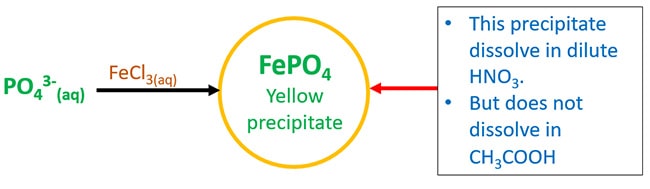
Testing of phosphate ion with Ferric chloride
Ferric chloride (FeCl3) and PO43- react and give ferric phosphate (FePO4) which is a yellow precipitate. This yellow precipitate will dissolve in dilute nitric acid. But do not dissolve in acetic (CH3COOH) acid.
Testing of phosphate ion With Magnesia mixture
Magnesia is a mixture of of Magnesium chloride (MgCl2). ammonium hydroxide (NH4OH) and ammonium chloride (NH4Cl). It gives a white crystalline precipitate of MgNH4PO4.
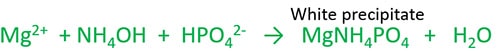
CrPO4 is a greenish precipitate.
Fertilizer Production and phosphate ion
Naturally occurring calcium phosphate(Ca3(PO4)2) is the primary resource utilized in producing fertilizer. But Ca3(PO4)2 is insoluble in water. Because PO43- ion is basic, it reacts with an acid to produce H2PO4- and it gives Ca(H2PO4)2 is more soluble than Ca3(PO4)2. Sulfuric acid is used as the acid.
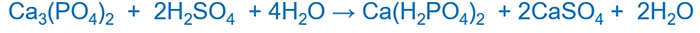
Now, we discuss about some phosphorus compounds and their reactions/
Oxyacids of phosphorus
H3PO4, H3PO3, H3PO2, H4P2O7 are oxyacids of phosphorus.
Phosphoric(V) acid (H3PO4)
Phosphoric acid is one of the chemicals produced in enormous(very large quantity) quantities, and it is used in many industrial processes. H3PO4 is not a oxidizing acid and a weak acid.
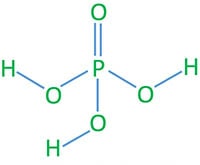
There are three salts of Na as NaH2PO4, Na2HPO4, Na3PO4,
Phosphoric acid preparing
P4O6 and water reaction.
P4O6 + H2O → 4H3PO3
PCl3 hydrolysis
PCl3 + 3H2O → H3PO3 + 3HCl
Phosphoric(III) acid (H3PO3)
H3PO3 is a weak acid and a dibasic acid. Oxidation number of P in H3PO3 is +3.
-acid.jpg)
Phosphoric(I) acid (H3PO2)
H3PO2 is a weak acid and a monobasic acid. Oxidation number of P in H3PO3 is +3. H3PO2 is an oxidizing acid.
-acid.jpg)
Occurrence of phosphate ion
Phosphate minerals include calcium phosphate (Ca3(PO4)2) , apatite(Ca5(PO42)3OH), fluoroapatite (Ca5(PO4)3F) and chloroapatite (Ca5(PO4)3Cl)
Questions
What are the yellow precipitates of phosphate ion?
silver phosphate - Ag3PO4