Amphoteric compounds, elements, metals characteristics
There are five amphoteric elements (metals) in the periodic table Be, Al, Zn, Sn, Pb. Also those elements oxides and hydroxides are amphoteric compounds. Amphoteric elements and compounds react with both acids and bases and give relevant products.
What is amphoterism?
The ability to react as an acid or a base, is known as amphoterism.
In this tutorial,
First, we discuss about amphoteric behavior of amphoteric different elements. Then we study about other amphoteric compounds of transition metals. Then explain some questions of amphoteric properties and compounds.
Amphoteric metals and their position in periodic table
All amphoteric elements are metals and they are located in s, p, d blocks.
- Be : s block (IIA group)
- Al : p block (IIIA group)
- Zn : d block (IIB group)
- Sn : p block (IVA group)
- Pb : p block (IVA group)
Amphoteric behavior of Be, Al, Zn, Sn, Pb
Now we discuss about amphoteric metals behavior of Be, Al, Zn, Sn, Pb and their oxides.
Amphoteric behavior of Beryllium
Beryllium is a metal of alkaline earth metals. Beryllium reacts with HCl and give beryllium chloride ( BeCl2 ) and H2 gas. Also beryllium reacts with NaOH and give sodium berrylate (NaBeO2.2H2O or Na2[Be(OH)4] ) and hydrogen gas(H2).
Beryllium and sodium hydroxide
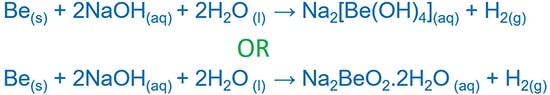
Beryllium and HCl

Amphoteric behavior of Aluminium
Aluminium reacts with both acids and bases to show amphoteric properties.
Aluminium reacts with HCl acid and give aluminium chloride ( AlCl3 ) and hydrogen ( H2 ) gas. Aluminium reacts with NaOH and give NaAlO2 or Na+[Al(OH)4]- which are aqueous solutions.
Aluminium and sodium hydroxide reaction
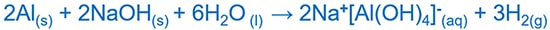
Aluminium and HCl
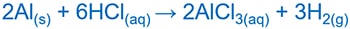
Aluminium hydroxide ( Al(OH)3 ) is also an amphoteric compound.
Amphoteric behavior of zinc
Zinc reacts with both acids and bases to give products.
Zn and HCl reaction

Zn and NaOH reaction
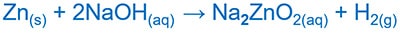
Amphoteric behavior of zinc oxide (ZnO)
ZnO can react as either an acidic or basic oxide and it is therefore known as an amphoteric oxide. ZnO reacts with HCl(aq), NaOH(aq), Na2O(aq).
Zinc oxide and hydrochloric acid reaction
Zinc oxide ( ZnO ) reacts with and hydrochloric acid ( HCl ) and give zinc chloride ( ZnCl2).

Zinc oxide and sodium hydroxide reaction
Zinc oxide ( ZnO ) reacts with sodium hydroxide ( NaOH ) and produce sodium zincate ( Na2ZnO2 ).
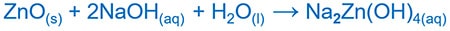
Zinc oxide and sodium oxide reaction
Zinc oxide ( ZnO ) reacts as an acid with sodium oxide ( Na2O ) and forms the zincate ion [Zn(OH)4]2- complex ion. This reaction is formally equivalent to the reaction without water.
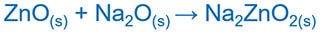
Amphoteric behavior of zinc hydroxide
Zinc hydroxide ( Zn(OH)2 ) is an amphoteric compound and a white precipitate. It reacts with HCl(aq) acid and give zinc chloride ( ZnCl2 ) aqueous solution. Also Zn(OH)2 reacts with excess NaOH and forms Na2[Zn(OH)4].
Amphoteric behavior of Lead
Lead(Pb) react with HCl and forms lead chloride ( PbCl2(s) ) and hydrogen gas ( H2(g) ). But Pb does not dissolve in concentrated HCl because a surface coating of PbCl2. Pb slowly reacts with cold alkali and rapidly by hot alkali forming plumbates.
Pb and HCl reaction

Pb and NaOH reaction
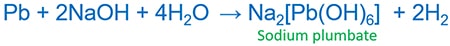
Amphoteric behavior of Tin
Tin(Sn) react with dilute HNO3 acid to form Sn(NO3)2, NH4NO3 and water. Sn react slowly with cold alkali, but with hot alkali Sn react rapidly for, stannates .
Tin and nitric acid reaction
Tin ( Sn ) reacts with nitric acid ( HNO3 ) and give tin(II) nitrate ( Sn(NO3)2, ammonium nitrate ( NH4NO3 ) and water as products.

Tin and sodium hydroxide reaction
Tin reacts with sodium hydroxide to give Sodium stannate ( Na2[Sn(OH)6] )

Amphoteric behavior of Beryllium ion
Beryllium oxide, Beryllium hydroxide exhibits amphoteric behavior.
Be(OH)2 and OH-
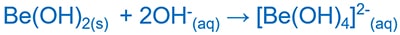
Be(OH)2 and H+
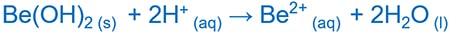
Amphoteric compounds of d block metals
Amphoteric characteristics of chromium compounds
Some 3d metal compounds such as chromium hydroxide, chromium(III) oxide, ferric oxide has amphoteric characteristics.
Amphoteric properties of chromium hydroxide (Cr(OH)3)
Chromium hydroxide (Cr(OH)3) is an amphoteric compound and a green precipitate. When NaOH(aq) is added that precipitate dissolve and give to [Cr(OH)4] - (aq) solution. Cr(OH)3 reacts with acids.
Amphoteric properties of chromium(III) oxide
Chromium(III) oxide (Cr2O3) is an amphoteric compound. It is green colour and reacts with both acids and bases.
Chromium(III) oxide and NaOH reaction
-oxide-and-NaOH-reaction.jpg)
Chromium(III) oxide and HCl reaction
-oxide-and-HCl-reaction.jpg)
Amphoteric behavior of ferric oxide(Fe2O3)
Ferric oxide(Fe2O3) reacts with both acids and basic oxides
Fe2O3 and acids

Fe2O3 and CaO or Na2CO3
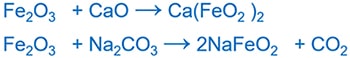
More points of amphoteric compounds
- Ga2O3 shows amphoteric characteristics.
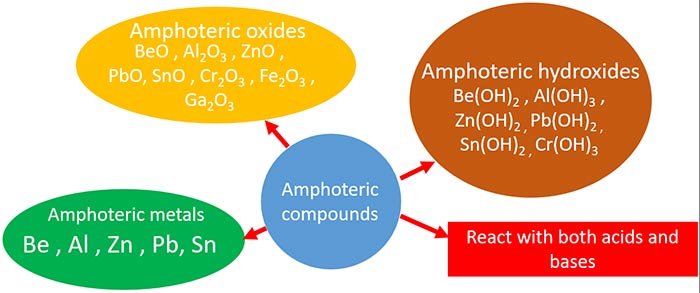
Amphoteric compounds, elements, metals summary
Below figure shows the summary of amphoteric compounds such as oxides, hydroxides and amphoteric metals.
Questions asked by students
Are all amphoteric elements are metals?
Yes. All amphoteric elements are metals.
Is NaOH amphoteric?
No. Alkali metals' hydroxides, oxides do not show amphoteric properties. Therefore NaOH is not an amphoteric compound.
Why do amphoteric oxides form complex compounds?
Amphoteric compounds form complexes with water or alkalis. Also you have to know, Not only amphoteric compounds form complexes. If metal ion is capable of forming complexes, there is no need to be a complex compound to form coordination complexes. All transition metals form complex compounds.
Alkali metals have less ability to form complex xompounds due to their less charge (+1).
>All 3d block elements are amphoteric in nature
NO. Zinc is the only amphoteric 3d block element.
location of amphoteric hydroxides in periodic table
As amphoteric metal existing locations, amphoteric metal hydroxides are located in amphoteric metal locations because all amphoteric metals' hyroxides are amphoteric. Instead of that, 3d metal chromium form a amphoteric hydroxide Cr(OH)3.
Related Tutorials of Amphoteric Compounds