Industrial and Domestic Soap Production and Manufacturing Process
Soap is an item of daily necessity as a cleaning agent. Soap production is one of the large chemical industry because it has a high demand in every part of the world. There are four basic raw materials involved in the manufacturing of soap and also there are three basic process methods that are used industrially; cold process, hot process and semi-boiled process. soap production is mainly done in four steps, saponification, glycerin removal, soap purification and finishing. We will discuss these things in detail in this tutorial.
Written by: Shashika Madusanka Gamag , (undergraduate), Department of Chemical and Process Engineering, University of Peradeniya, Sri Lanka
What is Soap?
Soap is an item of daily necessity as a cleaning agent. It is the traditional washing compound. Soap is a salt of high carboxylic acid. Basically, it made from oil fats and caustic alkali. By today, there are lots of soap types on the market. Among them, washing soap, medicated soaps, toilet soaps, kitchen soaps, and baby soaps are few especially soaps. Population growth is mainly impacting the growth of the manufacturing sector of the industry.
History of Soap
There are various legends surrounding the beginning of soaps but no one exactly knows when soap was discovered.
We can find the emergence of the first soap or at least the first use of soap in the Roman legends. According to Roman legends, soap was named after Mount Sapo, an ancient site of animal sacrifices. After an animal sacrifice, rain would wash animals' fat and ash, that collected under the ceremonial altars, down to the banks of the Tiber River.
Women washing clothes in the river noticed that if they washed their clothes in certain parts of the river after a rainfall their clothes were much cleaner.
Also, the earliest known written soap recipe was written on clay tablets and is credited to ancient Babylonians. From then on, it has evolved into the commercial soap we know, undergoing various processes and changes.
Raw Materials of The Soap Production
Mainly, four basic raw materials are involved in the manufacture of soap.
- Oils and fats
- Soda lye or potash lye
- Brine (for glycerin recovery)
- Additives (sodium carbonate, sodium silicate, dyes, perfumes, etc.) as secondary products.
Oils and Fats
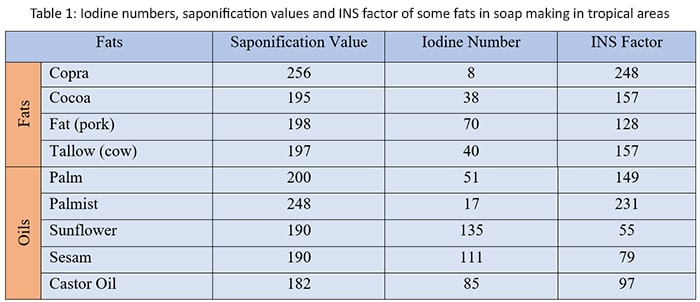
The natural fats used for soap making are triglycerol. Determines the suitability of triglycerol for soap product saponification using two main chemical parameters. They are explained below.
Iodine Number
The Iodine number of a fat express the weight of Iodine in grams which can be fixed from 100g of them. It shows the presence of double links, thus the degree of instauration of the carbonic chain. Its value varies between 10 to 200. Soap made from high Iodine Number fat has the tendency to be soft.
Saponification Value
The Saponification Value of a fat is the quantity of caustic potassium (KOH) in mg needed to transform 1g of it in soap. An easier transformation of the fat into soap indicated by a high Saponification value.
The INS factor (Iodine Number-saponification Value), which represents the difference between these indicates, is an essential parameter and is characterized as a soap derived from fat (mixture).
With increasing value of this factor, the following properties can be observed.
- The fat tends to be solid
- The soap tends sharply to become rancid
- The solubility of soap tends to decrease
- The soap made are rough
- The bleaching and forming properties tend decrease
Indicates the value of iodine and saponification indexes of the some of the main fats in soap making in tropical areas in the below table.
.
Coconut oil is extracted from copra. Copra has a range of pale yellow to white colors. It has very hard crumbly consistency. Copra has less stable foaming capacity and strong, quick foam characteristics. Copra is very good even at cold bleaching. It is rough effect on the skin. Soap of made by copra used for washing
When talk about Palm oil, it has yellow pale color. It has very crumbly consistency like copra. But palm has stable foam capacity and strong, slow foaming characteristics. Palm has very good bleaching effect and it is very smooth to skin. Soap of made by palm used for body hygiene and washing.
Lauric acid, which is found in high proportions in coconut oil and palm kernel oil, is the most commonly used fatty compound in soap with the best-expected properties. They are used in multiple formulas because they provide high lathering power and detergent. For cold or hot compaction, in combination with other oils and fats, it is used to improve hardness and reduce the dissolving speed of the manufactured soap.
Tallow and palm oil, usually after bleaching and deodorization, are the fatty materials most often used in combination with lauric acids.
Soda lye or Potash lye
We need to put strongly basic solution (alkali) in the water for direct saponification of neutral fats (triglycerols). Caustic soda and caustic potash are most used for this goal.
Caustic soda | Soda lye (NaOH)
Sodium soaps are harder and less soluble than potassium soaps, and are the most commonly used alkali for neutralizing fat due to its ability to return air humidity.
The concentrated caustic soda solutions are harmful to the skin and can cause serious burns.
Caustic potash | Potash lye (KOH)
It is a strong base, which like caustic soda, allows direct saponification of neutral fats. Potassium hydroxide is a type of lye specifically used to make liquid soap, and also, KOH is used in baby soaps because it is more environmentally friendly, water-soluble, and gentle for babies.
Brine
A concentrated brine solution is added to separate the glycerin from the soap. Soap made from fats such as copra, palm, or castor oil contain high levels of salt. In this case, salt can be used as a filler material. Also, the addition of salt to soda soaps can lead to very strong soaps. This method is based on the fact that saltwater is not soapy.
Additives
Additives are used for a variety of purposes and to enhance the quality of soaps. Adds pigments, fragrances, preservatives, fillers, etc. as additives. We will talk more about this later.
The Chemistry of Soap
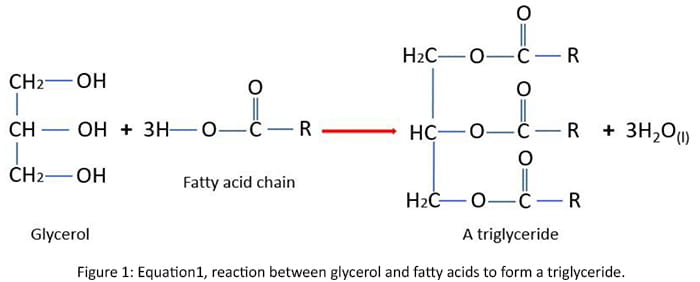
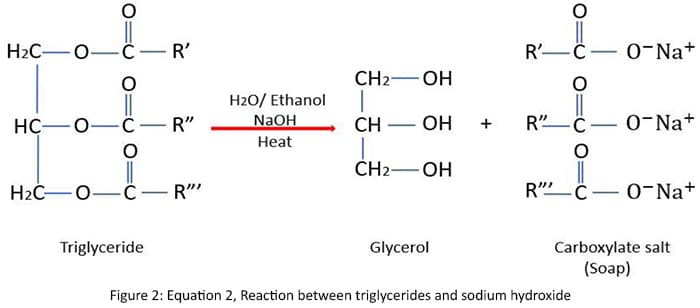
Soap is made by hydrolyzing a triglyceride using an alkaline solution. Triglycerides are typically triesters consisting of three long-chain aliphatic carboxylic acid chains appended to a single glycerol molecule. Sodium hydroxide (NaOH) is commonly known as lye.
The process involves heating the animal or vegetable oil in the lye. Carboxylate salts and glycerol then combine with the cations of the hydroxide compound to form a hydrolyze.
Equation 1 shows the reaction between glycerol and fatty acids to form a triglyceride.
Equation 2 shows the general reaction between triglycerides and sodium hydroxide.
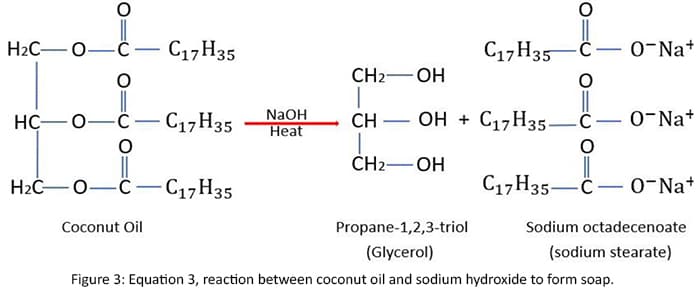
Equation 3 shows an example of a specific reaction between coconut oil and sodium hydroxide to form soap.
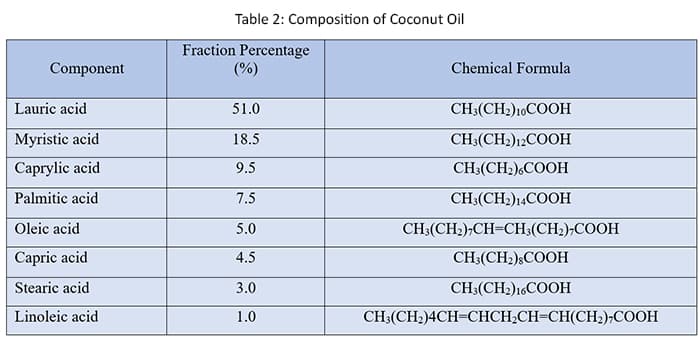
In general, coconut oil contains a wide variety of chemical compounds.
Soap Manufacturing Processes
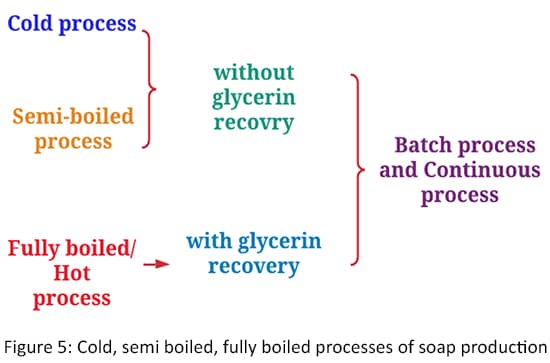
Considering the soap manufacturing processes, there are three basic process methods that are used industrially.
- Cold process (the reaction takes place substantially at room temperature)
- Semi boiled process (the rection takes place near the boiling point)
- Hot process/ Fully boiled process (the reactors are boiled at least once and the glycerol is recovered)
These three processes differ mainly in the saponification temperature. We will discuss more about this in the saponification step. From these processes, the cold process and the hot process are the most commonly used. In these three processes, soap production is mainly done in four steps.
- Saponification
- Glycerin removal
- Soap purification
- Finishing
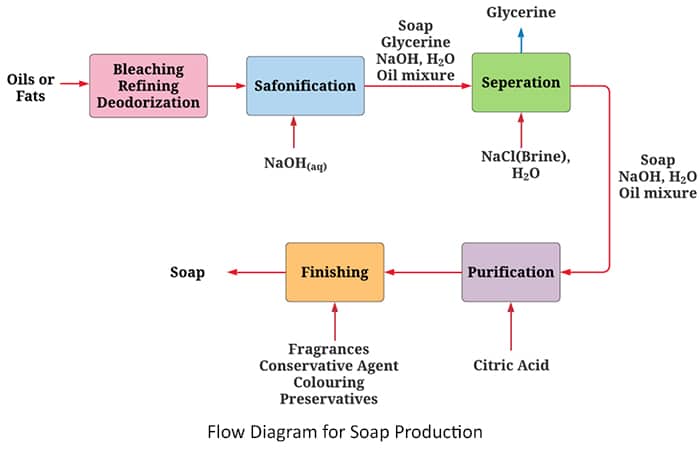
But first, need to clean the oils and fats are used as raw materials.
The Pretreatment of Oils and Fats
In general, some fats have strong odors and more or less intense colorations when raw state. Soaps made from these materials are of poor quality. Oil is purified by following treatments.
- Bleaching
- Refining
- Deodorization
Bleaching
Ingredients such as palm oil require some bleaching when making soaps such as toilet sap. But the majority of good quality oils and fats do not require bleaching.
One method is to send the oil through an "Active fuller iron" clay at a temperature of 90 degrees Celsius. Here the dirt, pigments etc. are removed. The clay particles in the oil are then removed. Similarly, bleaching is carried out by oxidation of oil, which is obtained through a stream of hot air at high temperatures.
Refining
This is a technology that is rarely used in the manufacture of pure soaps to refine the oil by treating it with alkali to remove free fatty acids.
Deodorization
This is a costly process, in which a superheated vapor stream passes through the oil.
Considering the three processes of making soap, the hot and cold processes, the first of these three processes is saponification.
Saponification in soap manufacturing
Let's first consider the general process of saponification step.
The refined oils or fats with soda lye ( NaOH(aq) ) or potash lye ( KOH(aq) ) in the reaction chamber. Sodium hydroxide or potassium hydroxide granules are not used here and only aqueous solutions are used. First sodium hydroxide is taken and dissolved in water. Here, too, some heat is generated and released. Similarly, when sodium hydroxide reacts and becomes inert, heat is released freely.
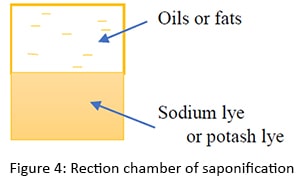
In the reaction chamber, the reaction chamber consists of two layers of sodium lye or potash lye and oils or fats. The interface here reacts to lye and oils or fats. In this reaction chamber, there is a constant stirring of reactors. It breaks down into small droplets in the reactant, increasing the surface area of the reactant molecules. At this point, the reaction rate increases as the surface area of the reactors increases.
Then the saponification process takes place quickly and effectively.
Soap production can take place in three main processes, as mentioned earlier, depending on the temperature at which the saponification takes place in the reaction chamber. Let's now talk about those processes which are cols, semi-boiled and hot processes.
Saponification: Cold Process
As the name implies, heat is not used to make soap. In the hot process, the soap is cooked at about 900C and then the glycerin is removed. But in the cold process the soap is saponified at room temperature and does not heat or wash. That is, the removal of waste or the removal of glycerin produced in not included in this process. The cold process is the most elementary batch process.
This process is a relatively long process compared to the hot process. The mixture is kept vigorous agitation for approximately 2 hours, and the dyes, perfumes and additives are generally added at this stage. As soon as most of the mixture has solidified, the crude soap is removed and poured into cooling frames. There, too, the saponification process continues for a day or more. The crude soap (fat content: 58%) is then removed from the frame, cut into chunks, and sent to the finish line.
It is advisable to use 1/3 of coconut oil or palm kernel oil to facilitate filtration and emulsification of fatty acids and to facilitate saponification to prevent contamination. This process is a simple, inexpensive method and is not highly mechanical.
Saponification: Semi-Boiled Process
This process differs from the cold process in that it uses a heated coil to heat the saponification mixture to a temperature of 70-900C. This causes the saponification reaction to complete faster.
This process allows the amount of baking soda to be adjusted before removing the dirty soap. The process also allows for better recycling of product waste, better integration of additives, and wider selection of raw materials. The semi-boiled process does not discharge any effluent into the environment.
Saponification: Full Boiled Process / Hot Process
This process is slightly different from the semi-boiled process. In this process, the saponification reaction usually takes place at temperatures as high as 1000C. Here, too, the fat ingredient allows for a wide range of uses. The glycerin is then separated from the soap by rinsing with alkali. We will discuss that at later.
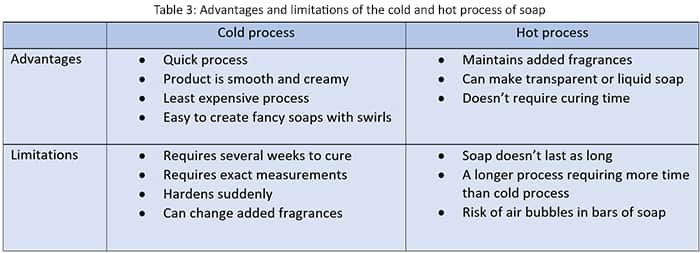
This process allows the production of a wide range of soaps, from basic household soaps to high-grade toilet soaps. we can identify some advantages and limitations of the cold and hot process of soap.
Advantages and limitations of the cold and hot process of soap
Glycerin Recovery in Soap Production
The final mixture obtained after saponification contains two parts, a solid zone, and an aqueous zone. Considering the aqueous zone, its alkalinity is low because of the reaction of sodium hydroxide. Also, glycerin is dissolved in aqueous phase. Also, a small amount of soap is dissolved and slightly ionized. Glycerin is more expensive than soap, so glycerin is removed. The amount of glycerin left over gives the soap a smooth and soft texture. Most of it is set aside for the production of more value-added beauty products.
The mixture obtained after saponification is in the following equilibrium.

If Na+ is added to the aquifer, the equilibrium reaction is reversed to minimize Na+ according to the Le Chatelier's principle. At this point, the density of the glycerol increases and the density of the soap decreases. The soap then rises to the top and glycerin deposited on the bottom. Glycerin is recovered from the bottom of the tank. Here the glycerin is separated by a difference in density.
A concentrated brine solution is usually added to the aqueous zone as a Na+.
Soap Purification
The mixture obtained by removing glycerin further contains impurities such as H2O, NaCl and NaOH. Therefore, in this step, the resulting mixture is centrifuged. That is, the mixture is further rotated with the impurities at high speed in a perforated vessel. It removes most of the water and salt that are the main product. But the final soap product contains about NaCl 0.5% (w/w).
There may be further unreacted sodium hydroxide. Sodium hydroxide can cause itching of the skin and damage to the delicate tissues of the eye. Therefore, any remaining sodium hydroxide should be removed.
Sodium hydroxide can be removed by neutralizing existing caustic soda by adding a weak acid such as citric acid. In addition to citric acid, phosphoric acid can also be used as a weak acid for this process.
Finishing
By cleaning the soap (the soap from the third step) is heated to a temperature of about 1200C. Then, the soap is sprayed into a low-pressure chamber. Next, the water in the soap particles evaporates. That is the temperature of the soap decrease by absorbing the temperature of the soap particles. Then the soap is deposited. That is, dry soap is produced. Dry soap is about 12% (w/w) water. Next, the evaporated water is removed. Fragrances, fillers, pigments, preservatives, etc. are then added.
Adding Salt to soap
Hard soap can be made by adding salt to soap. That is, it can be used as a salt filler.
Additives in soap
These products are added to soap in order to either increase the quantity or to give it a hard consistency. For that purpose, usually added kaolin or clay or starch or silicates of soda or potassium etc. Also, for produce transparent soaps, add additives like alcohol, sugar, and glycerin.
Adding colors to soap
Various chemical compounds are used to color soaps. Natural pigments can also be used. Among them can use the extracts from niebe leaves (vina sp) for the green color and extracts from red sorgo (Sorghum sp) for colors going from red-brown to orange.
Scents
The use of various chemical compounds and uses the extract from plants such as citrus and limes used as fragrances.
Skin protecting agents
Soaps made from certain fats are harmful to the skin. For that reason, it is necessary to add skin protection agents. For this purpose, added natural wax (about 3-5% of beeswax for example).
Industrial processes can actually be classified based on the process' output as:
- Continuous process
- Batch process
Let's consider these two processes.
Continuous Process
Here the main reactants or raw materials is continuously supplied to the reactor or mixer. Then products are given continuous even after a certain reaction or process. Continuous transport for many applications saves costs, energy and time. Here a large number of products are obtained. This method is often used in industrial production.
Batch Process
A process consisting of one or more sequences of steps to be performed in a specific sequence. Here the raw material is supplied all at once and the results are given after a reaction or a certain process. This process must be repeated. A whole unit of products is produced at one time.
The above all processes can be finally represented in the soap manufacturing process.