Ceramics | Crystal Structure, Characteristics, Imperfections, Applications
Ceramic is an inorganic non - metallic material which is formed in a firing cycle at high temperature. They are composed of two or more metals. Therefore, the structure of ceramics are more complex than other metals. Ceramics which we used in ancient time was known as traditional ceramic that includes pottery, clay products, (tiles, bricks) cement and silicate glasses. Ceramics that we use nowadays are called advanced ceramics, Technical or Engineering ceramics and Fine ceramics.
Most ceramic compounds exist between metallic and non- metallic elements. Therefore, interatomic bonds in ceramic are between ionic or predominantly ionic that have some covalent characters.
Written by: P.M.Thishini Nimesha, (undergraduate), Depatment of Civil Engineering, Faculty of Engineering, University of Peradeniya, last update: 06-06-2021
Importance to study on ceramics
In the Engineering field, it is very important to have a clear idea about the properties and their nature of metals, polymers, ceramics, etc. It is important for Engineers to realize how to influence the mechanical, thermal and other properties of ceramic according to the particular purposes. For an example, if someone wants to change the melting temperature they should have the knowledge on the atomic/ionic bonding and crystal structure of the ceramics.
Crystal Structure of Ceramics
In ceramics, those compounds materials exist as electrically charged ions instead of atoms. Therefore, it has an ionic bonding. The magnitude of the electrical charges on each ionic components and the relative sizes of those positive and negative ions are directly affect for the crystal structure and its properties. Also the whole structure should be electrically neutral in ceramics.
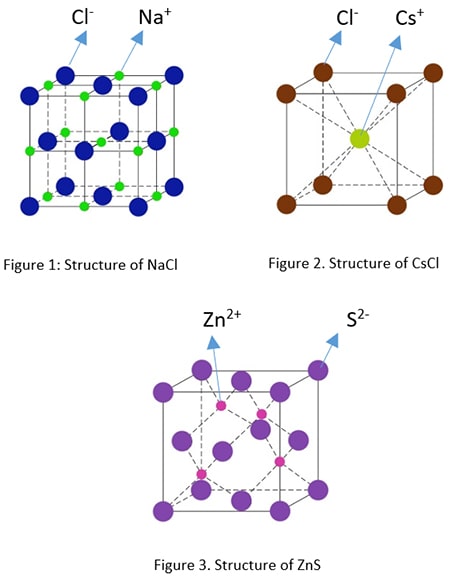
A-B Type Crystal Structures
Several examples given below.
- Rock Salt Structure (NaCl)
- Cesium Chloride Structure (CsCl)
- Zinc Blend Structure (ZnS)
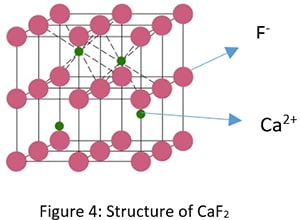
Am- Bn Type Crystal Structure
Examples: Fluorite ( CaF2), ZrO2 , UO2, PuO2
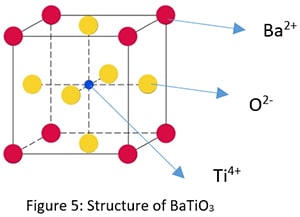
AmBnCp Type Crystal Structure
Example: Barium Titanate (BaTiO3).
Typical characteristics of ceramic
- Most specific property of ceramics is strong binding between atoms (covalent or ionic mainly).
- It has a high elastic modulus which is 2-3 times greater than that of metals.
- As it has a strong atomic bond, melting or dissociation temperature of ceramic is higher.
- Ceramics have a low atomic weight and a low density.
- Low chemical reactivity (Chemically inert).
- It has a high resistance to creep deformation.
- Ceramics are Brittle and hard.
- It needs high wear resistance and high stresses to have dislocations.
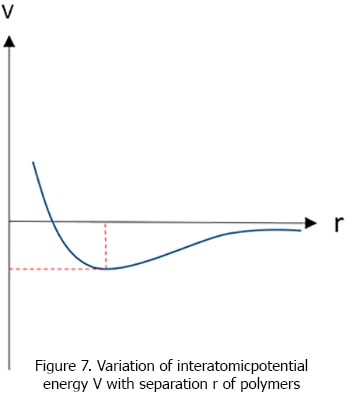
According to above curves, we can see that the depth of v - r curvature is higher in ceramics than the relevant curve of polymers. Therefore the Melting temperature of ceramics is greater than that of polymers.
Imperfections in Ceramics
Atomic defects can be happened among host in ceramic compounds. Both vacancies and interstitials are possible as it contains ions. Therefore, defects for each ion type can occur. There are two types of defects that can happen in ionic structures as Frenkel defect and Schottky defect. Even though in this incident, conditions of electro neutrality must be maintained. That means whatever defect type happens, it should contain equal numbers of positive and negative charges. We can influence the mechanical and other properties by changing those defects.
Applications of Ceramics
There are many different ways to classify ceramics. One way is we can define ceramics by considering their chemical compounds such as oxides, carbides, nitrides, sulfides, fluorides, etc. Another way is we can use to classify ceramics is by their major function. Today ceramics are widely used in many fields as refractories, spark plugs, dielectrics in capacitors, sensors, abrasives etc.
Pure Oxide Ceramics
Ex: Alumina(Al2O3 ) , Magnesia(MgO), Zirconia(ZrO2 ), Thoria (ThO2 ) , Beryllia(BeO), Titania(TiO2 )
Alumina (Al2O3 )
Alumina is one of the most commonly using ceramic which has the ability to bear high temperature and also has a high strength. It is used for car spark plug insulators as it has the electrical insulating property and high mechanical strength against spark corrosion. It is widely used as transparent lamp envelopes such as high intensity Na lamps. Most of wear parts (seals and valves) and cutting tools are made of alumina. Engine seals, Combustor, Turbine Nozzles and Oil pump rotor ring are some of them.
Electro - ceramics
Electro ceramics can be categorized into two. One is electrical insulators which are made of clay based ceramics called electrical porcelains. Other one is conducting ceramics such as SiC, MoSi2 and ZrO2 (Zirconia Ceramics)
Silicon Carbide (SiC)
Silicon Carbide exists in two polymorphic forms as α and β SiC structures. α form is in Hexagonal Closed Packed structure and β form is in Cubic Closed packed Zinc blend structure. This material is most widely used in structural ceramic. It has a relatively low thermal expansion, high specific modulus, high thermal conductivity and resistance to abrasion and corrosion. Its hardness considerably high. It has the ability to maintain its elastic resistance up to 16500C. This quality has affected for wide range of uses. Therefore it is used to make heating elements for furnaces. It also plays the role of a semiconductor.
Zirconia Ceramics (ZrO2)
This material exhibits in three types of polymorphs. They are monoclinic, tetragonal and cubic phases. The monoclinic is stable up to about 11100 C temperature. Then it transforms to the tetragonal phase at higher temperature and it remains stable up to 23700C. The cubic phase is stable up to the temperature of 2680 0C. Following are the most common applications of zirconia.
- We use this material to make cutting tool tips and abrasive wheels.
- Kitchen knives, blades for paper industry, surgical instruments are made of zirconia as it is corrosion resistant.
- It is widely used to make seals and valves which are really useful in chemical industry
- Refractory materials, ZrO2 refractory fibers and thermal barrier coatings are also made of zirconia.
- Zirconia also can be used as a colouring agent to make tiles, tableware, sanitary ware etc.
Super Conducting Ceramic
Ceramic materials including YBa2Cu3O7-δ , Tl-Ba-Ca-Cu-O and Hg-Ba-Ca-Cu-O show no resistance to electrical conductivity
below the critical temperature. Also these materials show meissner effect.
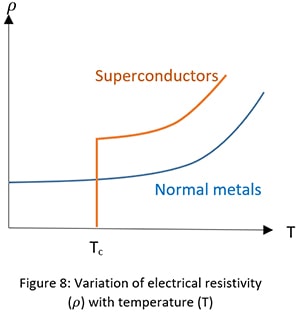
The critical temperature of super conductors are higher than that of normal metals. As there is no joule heating occurs high currents are possible through ceramic super conductors. Therefore, these are used in power transmission, strong electro magnets, some medical applications such as Magnetic Resonance Imaging (MRI), motors, dynamos and also in levitated trains
Magnetic Ceramics
Most of Ferrites which has the formula of MFe2O4 where M can be Ni, Mn, Zn, Cu, Co or mixture. These are used in large computer and memory devices.
Ceramic Nitrides
Ex : Si-Al-O-N (sialon), BN (Boron Nitride), TiN, AlN, Si3N4
Silicon Nitride Ceramic (Si3N4)
The atomic structure is formed by SiN4 tetrahedral units. These tetrahedral units are linked together in three dimensional framework by sharing corners. The bond between Si and N is covalent and short. Therefore they are very strong and it has affected to enhance its properties. So those ceramic materials have High strength and stiffness. Also good wear and corrosion resistance have increased the usage engineering applications.
Si-Al-O-N (Sialon) Ceramics
Sialon can be obtained by substituting Al for Si and O for N in Si3N4 (The net charge neutrality remains unchanged here). The polycrystalline micro structure of sialon has very low grain boundary where the grain size is about 1 µm. This ceramic type has low density and high strength which is resistant for thermal shock also. High wear resistance, high creep resistance and high oxidation resistance are some of other properties. These are used in many areas such as extrusion and wire drawing dies, Nozzles, Seals and molten metal containers and pipes etc.
Piezoelectric Ceramics
When we apply a strain to some types of ceramics, they tend to polarize. So one side of this polarized crystal take a positive charge and the opposite side become the negative charge. This effect is known as piezoelectric effect. The Greek term piezoelectricity gives the meaning of a combination of pressure and electricity. It is used in the applications where pressure results in a measurable electrical potential.
Quartz, Lithium Sulphate, Cadmium sulphide are some of the examples of piezoelectric ceramics. These type of ceramics are widely used in ultrasonic devices, microphones, earphones, ultrasound generators, detectors which are used in medical scanners, accelerometers, sonar devices etc.
Problems with Ceramics
Although ceramics have unique properties which are really used in many fields, it have some failures or problems too. Ceramics are brittle and difficult to fabricate, form or sinter. Therefore we have to use ceramic composites and should use new fabrication, forming and sintering methods to avoid that barrier. Also machining is difficult in ceramic and they are somewhat very expensive.
When we use ceramic for applications, we have to use many methods to join two or more materials. But here normal welding methods cannot be used for ceramics. Therefore we have to look for new technologies when we work with ceramics.
Requirements to start a ceramic production plant
If we want to start any kind of a plant we have to follow basic steps by fulfilling the basic requirements. Generally we have to consider some facts such as how to demand our product, way to setup cost for the product, How to compete other manufactures etc.
In a ceramic product plant, after identifying a place basically we should have an idea on what we are going to manufacture. We need to identify basic raw materials such as silica, sand, quartz, flint, silicate ect. It will be better if we can reach to those raw materials easily from the plant. It is very important to have a deep knowledge on new techniques which can be used in the plant and we should have related machines to start a plant.
Also we need to identify suppliers and buyers of our product. It is compulsory to identify the legal requirement such as registration, compliance ect to keep our plant at a good state. After starting the plant we should look for new methods regularly that we can use to expand our product plan.
Leading ceramic manufacturers in the world
- The American giant Mohawk Industry - Ceramic tiles
- Lamosa and Vitromex in Mexico - Ceramic tiles
- Zimbo Qimingxing new material incorporated Co.LTD – Ceramic grinding media, Ceramic lining, Grinding equipment
- Kiyocera Corp in Japan – Electronic and Semiconductor materials
- CeramTec North America – Oxide/Non oxide ceramics and composite ceramics
- Fujimi Corporaion in Tualatin – Offers chemical and other products for lapping, grinding, polishing
- ACI Alloys in San Jose – Offers ceramics nitrides, oxides, fluorides, sulfides and chlorides
- HSIL, India - Ceramic tiles
- Magnetic component engineering Inc in Torrance – Coating and Planting, Assemblies, Magnetizing, Testing, Marking, Calibration, and Stabilization of many materials including ceramics
- Susan Jablon Mosaics, United States - Tiles
- Ann Sacks, United Stated – Ceramic tiles and deliver recycled glass and energy efficient concrete