Alkyne and Grignard Reagent Reaction
Alkynes which have terminal hydrogen atom react with grignard reagent and give an alkane as the product. Grignard reagent is a strong nucleophile and a base. Therefore, they can take protons from acidic compounds. Finally, grignard reagent is destroyed due to reaction with alkyne which has terminal hydrogen atom.
We consider grignard reagent as CH3MgBr.
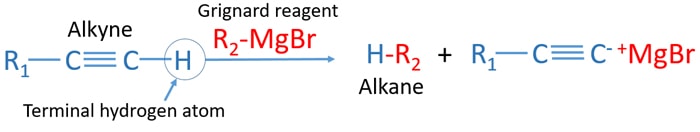
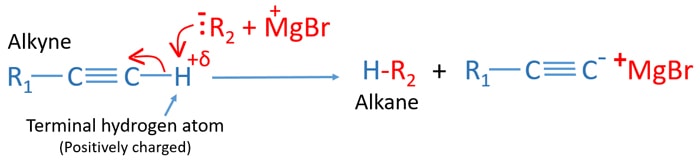
Mechanism of alkyne and grignard reagent reaction
- Terminal hydrogen atom (acidic hydrogen atom) has a small positive charge. Because of that, those kind of alkynes shows weak acidic properties.
- Grignard reagents are strong nucleophiles and they like to attack positively charged parts like terminal hydrogen atoms.
- Now, negatively charged alkyl group of grignard reagent attacks terminal hydrogen atom and takes it to form an alkane.
- With that, the bond between carbon atom and terminal hydrogen atom is broken. Electrons of the bond are taken by carbon atom and a carbanion is formed as a result.
- +MgBr part is reached towards anion of alkyl group and form an ionic bond.
Examples of reactions of alkyne which has terminal hydrogen and grignard reagent
Acetylene (ethyne) and CH3MgBr reaction
Acetylene gas two terminal hydrogen atoms. With methylmagnesium bromide (CH3MgBr), acetylene reacts and methane is produced by taking a terminal hydrogen atom. If, in the presence of excess CH3MgBr, other terminal hydrogen bonds reacts with CH3MgBr and produce more methane.
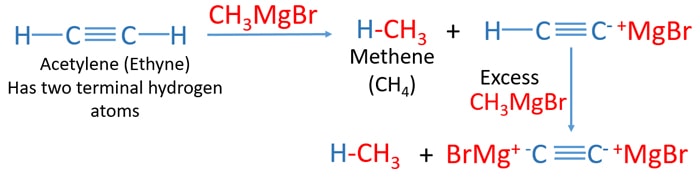
Finally, grignard reagent is destroyed. Therefore, grignard reagent cannot be produced when respective alkyl halide compound which contains a terminal hydrogen atoms connected to a alkyne group. Produced grignard reagent react with with terminal hydrogen atoms of own grignard reagents's