Calculate pH of Carbonic Acid (H2CO3) | Examples | Online Calculator
Carbonic acid (H2CO3) is a weak dibasic acid. That means, aqueous carbonic acid solution contains very low H+ ion concentration compared to the undissociated H2CO3 acid concentration.
In this tutorial, we will discuss following sections.
- Dissociation of carbonic acid acid
- Calculate pH of carbonic acid by using dissociation constant (Ka) values
- Online calculator
- pH values of aqueous carbonic acid solutions in different concentrations
Dissociation of carbonic acid
Carbonic acid is a dibasic weak acid. It means, there are two hydrogen atoms which can release as H+ ions in H2CO3 molecule. Though there are two releasable two H+ ions, only limited number of H2CO3 acid molecules releases H+ ions compared to the existing H2CO3 molecules.
- As the first dissociation step, H2CO3 molecule dissociates partially in water to bicarbonate ion (HCO3-) and hydronium ion (H3O+) ion. This reaction is reversible and generally the equilibrium point is shifted to the left side (In strong acids such as HCl, equilibrium point almost is shifted to right side).
- As the second dissociation, formed bicarbonate ions partially dissociates to carbonate ion (CO32-) and hydronium ion (H3O+) ion. Remember that, second dissociation is more weaker than first dissociation.
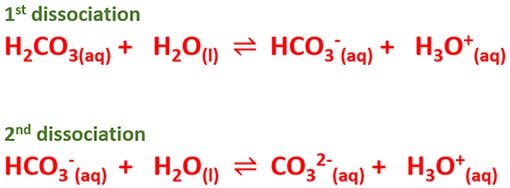
H2CO3(aq) + H2O(l) ⇌ HCO3-(aq) + H3O+(aq)
HCO3-(aq) + H2O(l) ⇌ CO32-(aq) + H3O+(aq)
Calculate pH of nitrous acid by using dissociation constant (Ka) value
After writing down the equation of dissociation reactions, expressions for each dissociation constants are written. Equilibrium concentrations of compounds and ions should be substituted to the terms in this equations to find H3O+ ion concentration and pH value.
Equations for dissociation constants of carbonic acid
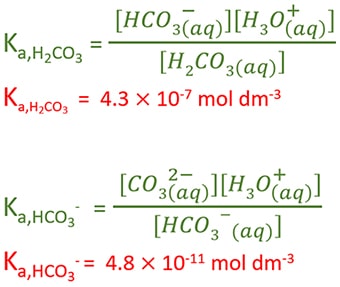
By comparing dissociation constants of carbonic acid, we can understand that second dissociation is much more weaker than first dissociation.
Equilibrium concentrations of each constituent are determined as following by creating tables.
Equilibrium concentrations of carbonic acid
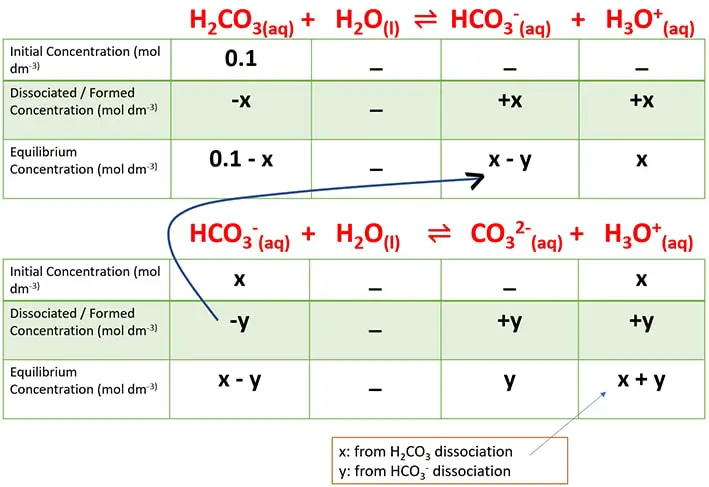
Assume that we are going to find the pH of 0.1 mol dm-3 nitrous acid solution.
Because second dissociation is more weak, we can suggest that considerable amount of H3O+ is only given by the first dissociation. Assuming like that, we can substitute known values into the equation as below.

Now we know the concentration of H3O+ and HCO3- ions. Next, we can try to find the concentration of CO32- ion.
Calculate carbonate ion concentration in carbonic acid solution
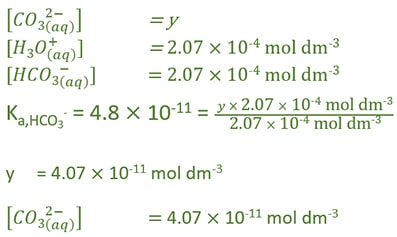
Find pH value of carbonic acid
In an earlier step, we have found that the concentration of H+ (H3O+) ions and substitute that value in pH calculation equation.
- pH = -log[H3O+(aq)]
- pH = -log[2.07 * 10-4]
- pH = 3.68
Assumptions made in calculations
- H3O+ concentration given by water is neglected because dissociation of water is much poor compared to the carbonic acid dissociation.
- Second dissociation is much weaker than the first dissociation and H3O+ concentration given from second dissociation is negligible.
Online pH calculator of carbonic acid solution
First dissociation constant (Ka) value of carbonic acid at 250C is taken as 4.3 * 10-7 mol dm-3. Also, second dissociation is neglected.
Answer
Try: weak acid pH online calculator
pH values of carbonic acid at different concentrations
| Concentration of carbonic acid (mol dm-3) | pH |
|---|---|
| 0.1 | 3.68 |
| 0.05 | 3.83 |
| 0.01 | 4.18 |
Questions
What is correct about pH of carbonic acid?
- Because there are two hydrogen atoms which can be released as two H+ ions, aqueous carbonic acid solution should show a lower pH value.
- Because carbonic acid is formed when carbon dioxide gas is dissolved in water, pH of that solution is enough to form acid rain.
- Because both hydrogen atoms poorly dissociates in water, carbonic acid is considered as a weak acid.
Answer: 3rd choice is the correct one. Carbonic acid is considered as a dibasic weak acid in chemistry. Carbon dioxide gas is not considered as a acid rain forming gas because carbonic acid is unable to generate very lower pH value when carbon dioxide gas dissolve in water.