Aniline and Nitrous Acid Reaction | C6H5NH2 + HNO2
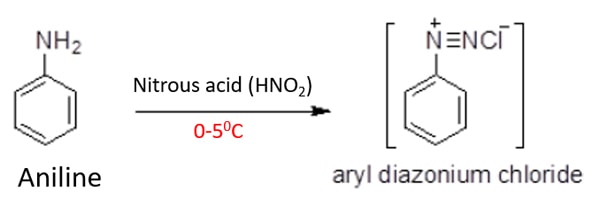
Aniline gives benzenediazonium chloride with aqueous nitrous acid (HNO2) at lower temperatures such as 0-50C. If aqueous solution is at room temperature, benzene diazonium chloride hydrolysis to phenol easily. Benzene diazonium is only stable in low temperatures.
Aniline and nitrous acid at room temperature
At room temperature, phenol is given as the product. As an intermediate product, benzenediazonium chloride is given. But, benzenediazonium chloride is not stable at the room temperature and readily decomposes to phenol. With that, nitrogen gas is produced too.
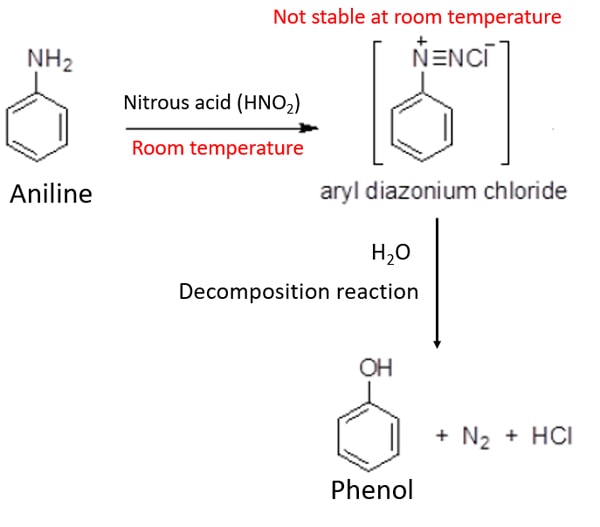
C6H5NH2 + HNO2 → C6H5OH + N2 + HCl
Then, how do I prepare benzenediazonium chloride?
Reduce the temperature to below than 50C. It is perfect to use 0-50C temperature. So, mix aniline and nitrous acid at 0-50C temperature. You will get benzenediazonium chloride. Remember that, you have to maintain that 0-50C temperature throughout the reaction and after the reaction to protect benzenediazonium chloride from decomposition.
What is nitrous acid>
Nitrous acid (HNO2) is prepared when you require it because we cannot store it for long time due to its spontaneous decomposition to nitric acid and nitric oxide (NO).
Uses of aniline and nitrous acid reaction
- One product, benzenediazonium chloride is used to synthesis dyes. They are called azo dyes.
- If phenol is made as a product, it has so many uses such as a disinfectant.
Questions asked by students.
Can I prepare benzene from aniline and nitrous acid reaction?
No. You will get phenol or benzenediazonium chloride according to the temperature of the reaction medium. When aniline (C6H5NH2) reacts with nitrous acid (HNO2) at room temperature, phenol is given as the product.
Is nitrogen gas emitted when benzene diazonium chloride reacts with nitrous acid?
If benzene diazonium chloride reacts with nitrous acid at room temperature, nitrogen gas is emitted.