Ammonia + Oxygen Reaction | NH3 + O2 Balanced Equation
Ammonia reacts with oxygen gas when heat is supplied and ammonia is oxidized as a result. Also this reaction occurs with catalyst and without catalyst under different conditions and you will learn everything about these reactions in detail in this tutorial.
At the end of this tutorial, you should know,
- Reaction of ammonia with oxygen without catalyst and how to balance the equation
- Reaction of ammonia with oxygen with catalyst and steps of balancing the chemical equation
- Uses of this reaction in industrial scale productions
Ammonia and oxygen without catalyst | NH3 + O2 → N2 + H2O
With supply of heat, ammonia reacts with oxygen and produce nitrogen gas and water as products. Nitrogen of ammonia is oxidized to nitrogen gas from -3 oxidation state to 0 oxidation state.

Balanced equation of NH3 + O2 without catalyst
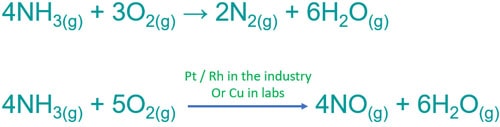
4NH3(g) + 3O2(g) → 2N2(g) + 6H2O(g)
Both ammonia and nitrogen gas are colorless gases. But their characteristics are so different.
How to balance the equation?
- This is a redox reaction (oxidizing - reducing reaction). You know the oxidation numbers of each element in the reaction
and identified which elements oxidation numbers are changed. Write oxidation numbers of those elements close to the each
element (See the figure).
- Balance elements which are oxidized and reduced in the reaction. Now, we want to balance nitrogen and oxygen atoms.
- There are two nitrogen atoms in the right side of the reaction while right side only has one nitrogen atom in the left side. So make two nitrogen atoms by adding two ammonia.
- In the left side, there are two oxygen atoms. But, in right side only one oxygen atom. Make two H2O to balance the oxygen atoms.
- Take the oxidation number differences for oxidation and reduction reactions. If those differences can be simplified, simplify them.
- Exchange those differences.
Ammonia and oxygen with catalyst | NH3 + O2 → NO + H2O
In this reaction, ammonia is oxidized to nitric oxide (NO) and oxygen is reduced to water. Oxidation number of nitrogen is increased from -3 to +2. This is the first reaction of nitric acid production.

Heat of reaction
This reaction is a exothermic reaction and it emits -904 kJ mol-1. This is a very large amount of heat and it is an advantage to continue the reactions furthermore to manufacture nitric acid,
Balanced equation of NH3 + O2 with catalyst
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
Released nitric oxide readily converts to nitrogen dioxide, a brown colour and very toxic gas.
2NO(g) + O2(g) → 2NO2(g)
Catalyst used in the oxidation of ammonia by oxygen
- In industrial scale, platinum, rhodium are used as catalyst because they are very much efficient.
- In the laboratory scale, copper is used because it is cheaper element than platinum and rhodium. But copper is less effective than platinum and rhodium.
Why catalyst is used?
This reaction is used in ostwald process which produces nitric acid from ammonia. In that process, higher temperature is also used to increase the reaction rate to get the yield in a short time.
When copper acts as a catalyst with ammonia, what is the role of copper oxide (CuO) with ammonia?
With solid CuO and supply of heat, ammonia is oxidized to nitrogen gas and CuO is reduced to copper. Copper oxide (CuO) is a black solid and produced copper has red-brown color.
3CuO(s) + 2NH3(g) → N2(g) + Cu(s) + 3H2O(l)
So CuO does not show a catalytic behavior with ammonia gas because amount of CuO is reduced when reaction is in progress. So Cu and CuO behave differently with ammonia.
Questions
Ask your chemistry questions and find the answerswhat is room temperature what kind of change takes place when ammonia burns in oxygen
If ammonia burns in oxygen at room temperature, nitrogen gas and water are formed when there is no catalyst. To oxidize nitrogen to nitric oxide, there should be high temperature. But, room temperature is not enough to do that oxidation process.
Can use NH3 + O2 reaction to generate power?
First think I should ask is, why do you use NH3 to generate power? There are so many fuels you can use for power generation. NH3 + O2 reaction gives lot of energy if it is used to henerate electricity. But, it is a waste because ammonia is much valuble than a fuel.
nh3 with o2 under cu catalyst?
This method is used to oxidize NH
What is the difference between reaction of oxygen and ammonia without catalyst and with platinum
Without catalyst, ammonia is only oxidized to nitrogen gas. But in the presence of platinum catalyst, ammonia is oxidized to nitric oxide.
Will ammonia and oxygen reaction give a toxic chemicals?
If ammonia react with oxygen without catalyst, nitrogen and water are given. Both compounds are not toxic.
Whem ammonia react with oxygen in the presence of Pt catalyst, nitric oxide (NO) and watwe are given. At levels of 100 ppm, nitric oxide is immediately dangerous to life and health.
NH3 O2 reaction
Oxidation of ammonia by oxygen gas can be done in several ways using different conditions. According to the reaction conditions, products also vary.
What happen to oxidation state of N in NH3
WHen ammonia is oxidized by oxygen, oxidation number of N in NH3 increases from -3 to a higher higher oxidation number such as 0 or +2. O oxidation number is for N2 gas and +2 for NO gas.
Why ammonia + oxygen reaction should be done carefully?
Ammonia is a hazardous gas and very toxic to humans. So when this reaction is conducted, ammonia should be handled very carefully to prevent any leakages to outside environment.
Is ammonia will be oxidized, when silver oxide is heated?
One of my friend told, ammonia is oxidized by silver oxide (Ag2O). Is this true? If it is possible, how it happens?
Yes. Ammonia gas can be oxidized to nitrogen gas by silver oxide. Silver oxide is not stable to heat and decomposes. When silver oxide is heated, oxygen gas and silver metal are given as products.
With produced oxygen gas, ammonia is oxidized as before seen in the tutorial.
oxidation of ammonia to nitric acid
Oxidation of ammonia in the presence of catalyst by oxygen gas to NO and furthermore oxidation of NO to NO2 by oxygen gas is used in nitric acid production.
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
2NO(g) + O2(g) → 2NO2(g)
2NO2(g) + H2O(g) → HNO3(aq) + HNO2(aq)
What will happen to hydrogen atoms in the ammonia, when reacts with oxygen.
Hydrogen atoms combines with oxygen gas and produce water. Oxidation number of hydrogen does not change in this reaction. In the reaction of hydrogen sulfide and sulfur dioxide, oxidation number of hydrogen in H2S does not change.
why reaction between ammonia and oxygen exothermic?
Exothermic reactions gives products which have less energy than reactants. When energy of a compound is low, that increases the stability. Therefore, rather than being ammonia and oxygen, they like to react with each other to produce low energy compounds.
Why ammonia and oxygen reaction is an advantage to nitric acid production.
Ammonia and oxygen reaction release very large heat and it is helpful to do furthermore reactions.
Oxidation number of nitrogen in ammonia
Electronegativity of nitrogen is higher than hydrogen. So electrons of three N-H bonds are attacked towards nitrogen atom. So oxidation number of nitrogen in ammonia is +3.