Sodium Hydroxide and Chlorine Gas Reaction | NaOH + Cl2
Aqueous Sodium hydroxide solution readily reacts with the Chlorine gas in two ways according to the concentration and
temperature of the solution and gives different products depending on those parameters.
Reaction between NaOH solution and Cl2 gas is an
Redox reaction (oxidation - reduction) and this reaction become more special because
this is a disproportionation reaction. In this tutorial, we will discuss these facts in detail.
Content
- Ways of reactions between NaOH and Cl2 gas according to the temperature and
concentration
- Reaction of cold dilute NaOH solution and Cl2 gas
- Products given by the reaction of cold dilute NaOH and Cl2 gas reaction
- Stoichiometric equation (Chemically balanced) of cold dilute NaOH and Cl2 gas reaction
- Reaction of hot concentrated NaOH solution and Cl2 gas
- Products given by the reaction of hot concentrated NaOH and Cl2 gas reaction
- Stoichiometric equation (Chemically balanced) of hot concentrated NaOH and Cl2 gas reaction
- Stability of Hypochlorite ion | Chlorate(i) ion | OCl-
- Other halogens with aqueous sodium hydroxide solution
- Stability of Hypochlorite ion | Chlorate(i) ion | OCl-
- Mechanism of Cl2 gas and dilute cold NaOH reaction
- Safety of NaOH and Cl2 gas reaction
- Detailed summary of products and characteristics of the Reaction
Ways of reactions between NaOH and Cl2 gas according to the temperature and concentration
There are two reactions can be happened according to the concentration and temperature of the sodium hydroxide solution.
- Cold dilute NaOH and Cl2 gas reaction
- Hot concentrated NaOH and Cl2 gas reaction
Cold Dilute NaOH and Cl2 gas reaction | NaOH + Cl2 = NaCl + NaOCl + H2O
In this section, we will discuss what products are given under specific conditions and the chemically balanced equation.
Products given by the reaction of cold dilute NaOH and Cl2 gas reaction
Cold dilute sodium hydroxide and chlorine gas reaction is disproportionation reaction.
Chlorine gas is oxidized and reduced to hypochlorite ion (OCl-) ion and
Chloride (Cl-)
ion respectively. As products ,Sodium chloride, sodium chlorate(i) and water are given.
- Hypochlorite ion | chlorate ion(i) - OCl-
- Sodium chloride - NaCl
- Sodium chlorate(i) - NaOCl
Aqueous solution which contains Sodium chloride (NaCl) and Sodium hypochlorite (NaOCl) is called as the Milton solution. Hypochlorite ion
is an active anion and exists in the bleaching powder.
Stoichiometric equation (Chemically balanced) of cold dilute NaOH and Cl2 gas reaction
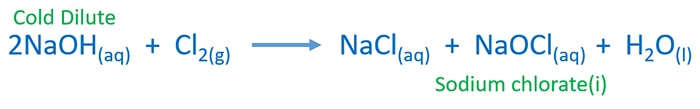
In hypochlorite ion, chlorine atom is in the +1 oxidation state. In NaCl, chlorine is at -1 oxidation state.
Hot concentrated NaOH and Cl2 reaction | NaOH + Cl2 = NaCl + NaClO3 + H2O
Products given by the reaction of hot concentrated NaOH and Cl2 gas reaction
As earlier reaction, Hot concentrated sodium hydroxide and chlorine gas reaction is also a
disproportionation reaction. Chlorine gas is oxidized and reduced to Chlorate (ClO3-) ion and
Chloride (Cl-) ion respectively.
As products, sodium chloride, sodium chlorate(iii) and water are given.
- Chlorite ion - ClO3-
- Sodium chlorate(iii) - NaClO3
Stoichiometric equation (Chemically balanced) of hot concentrated NaOH and Cl2 gas reaction

In chlorite ion, chlorine atom is in the +5 oxidation state.
Stability of Hypochlorite ion | Chlorate(i) ion | OCl-
Hypochlorite (OCl-) ion is an unstable ion it is stable only in cold state conditions. When temperature
increases, it disproportionate to chloride ion and chlorate ion. In Hypoclorite ion, negative charge is on the oxygen atom becuase
oxygen is more electronegative than chlorine.

Other halogens with aqueous sodium hydroxide solution
As chlorine gas, other halogens except
fluorine
also react with sodium hydroxide and give similar products as NaOH and Cl2 gas reaction.
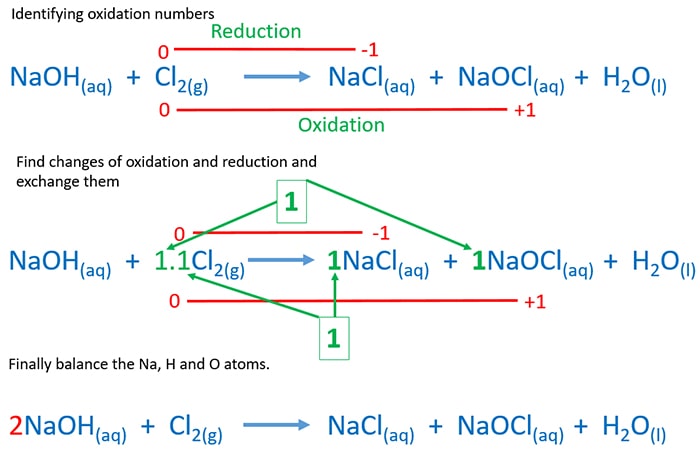
How to Balance the cold dilute NaOH and Cl2 reaction
- We know this reaction is a disproportionation reaction. When we see the products, it's clear that Cl2 is oxidized
to OCl- and reduced to Cl-. Oxidation numbers of chlorine in OCl- ion is +1 and Cl- ion is
-1.
- Now check the differences of each oxidation numbers in oxidation and reduction. It is one in both. Then exchange the differences.
- Then balance other elements. Check number of Sodium atoms in both sides. There are two Sodium atoms in the right side. So we make two number
of NaOH.
- Then check the balance of number of hydrogen atoms. Left side has two hydrogen atoms so far now. Then only one H2O molecule in the right side
is enough to balance the number of hydrogen atoms in the equation.
This type of redox reaction balancing example is reaction of chlorine gas with ammonia.
Mechanism of Cl2 gas and dilute cold NaOH reaction
When chlorine gas dissolve in water and reacts with
water, it produce hypochlorous acid (HOCl) and
hydrochloric acid (HCl). You know
acids react
with bases and produce a salt and water as products. So, when NaOH is in the presence of HOCl and HCl solution, following
reactions are occurred.
- HOCl and NaOH react and produce NaOCl and water.
- HCl and NaOH react and produce NaCl and water as products.
Safety of NaOH and Cl2 gas reaction
This reaction should be conducted in a safe manner to avoid any accidents because there are dangerous chemicals.
- NaOH is corrosive and may severely irritate skin, eyes, and mucous membranes. Also corrosive to the metals.
- Cl2 is a yellow - green colour and extreemely toxic gas to humans. When you dealing with Cl2, be very careful to
get protected from it. Specially, avoid any Chlorine gas leakages to outside environment.
Detailed summary of products and characteristics of the Reaction
Here, we discuss some special features and characteristics to this reaction.
A disproportionation reaction?
A reaction in which a substance is simultaneously oxidized and reduced, giving two different products. Reaction of NaOH and Cl2 gas
reaction, Hydrogen peroxide decomposition to water and O2 gas are two examples.
Production of NaOCl and NaClO3
Now we need to produce NaOCl and NaClO3. So we need NaOH and Cl2 gas.
Is something similar products will give Electrolysis of Brine (NaCl)
Electrolysis of brine solution will produce NaOH, Cl2 gas and H2 gas as products.
How to produce NaOCl?
We can collect Cl2 gas can cool the gas before send it to the produced NaOH to occur the reaction.
NaOH solution also should be cold to keep NaOCl stable.
How to produce NaClO3?
Produced Cl2 is sent to the hot concentrated NaOH.
In both occasions, NaCl is produced as a product. We can separate NaClfrom NaOCl or NaClO3 by a separation method.
Questions asked by students
Ask your question and find the
answer free.
As chlorine gas, will bromine and iodine react with sodium hydroxide?
We know bromine and iodine are less soluble than chlorine in water. Therefore, bromine and iodine do not react with sodium hydroxide as chlorine gas.
That means, their (bromine and iodine) reaction rates are much slower than chlorine gas.
equation for the reaction between chlorine and hot concentrated alkali
We can denote the alkali as MOH in the equation. Chlorine (Cl2) is oxidized to chlorate
(ClO3-) ion and chloride ion (Cl-) when it reacts with hot concentrated alkali.
3Cl2 + 6MOH → 5MCl + MClO3 + 3H2O
What will happen, when Cl2 gas is sent to the water?
Cl2 hydrolysis in the water. HCl and HOCl are given as products. HCl is a strong acid and HOCl is a weak acid.
HOCl is a very good disinfectant and more efficient than
bleaching powder.
Can I use this chlorine and NaOH reaction to neutralize Cl2 gas?
Yes. You can use NaOH and this is very efficient way to do the neutralization. But you have to consider about the cost of this
neutralization method because NaOH is so expensive.
What is chlorinated water or chlorination of water?
The process of adding chlorine or chlorine compounds such as sodium hypochlorite (NaOCl) to water. This is done in swimming
pools. This is done to kill certain bacteria and other microbes in tap water as chlorine is toxic to those microbes.
That's why we feel smell of chlorine gas in the swimming pools.
Chlorine reacts with hot and concentrated NaOH, But why products are different?
When temperature is high, the ability to oxidation is high. Therefore produced NaOCl is oxidized to NaClO3
from +1 to +5 (oxidation umber of chlorine).
How this reaction is different from sodium hydroxide and HCl reaction?
NaOH and HCl reacts as base and acid respectively and produce salt (NaCl) and water as products. That reaction is not a redox
reaction.
disproportionation reaction of water with sodium hydroxide
There is no disproportionation reaction oof water and sodium hydroxide. Only sodium hydroxide dissolve very well in water and
dissociate to sodium ion and hydroxyl ions completely.
OCl- ion oxidation state?
We know electronegativity of oxygen is higher than chlorine. So negative charge should be on oxygen atom whole chlorine atom
is oxidized due to higher electronegativity of oxygen. There is a single bond between oxygen and chlorine. Therefore oxidation number of chlorine is +1.
What will be the pH value after cold dilute NaOH reacts with chlorine gas if reactants are completely finished?
We know NaOH is a strong base.
So it shows higher pH value which is greater than 7 at 250C.
How pH is calculated for a strong base?
When all NaOH reacts with all chlorine, there is no remaining NaOH and Cl2. Produced NaCl and NaOCl are in the
solution and we should consider, whether their ability to hydrolysis of water to make water acidic or basic.
how does sodium hydroxide neutralize chlorine?
Use aqueous sodium hydroxide solution to neutralize chlorine gas. But commonly, spraying a solution of baking soda in water into
the air or urea solution will be used. Cost of NaOH is very high.
Equipment to react chlorine with caustic soda?
Chlorine gas reacts with iron and produce ferric chloride. So we cannot use iron as the reactant container equipment.
Also, caustic soda slowly reacts with
glass and form sodium silicate. So glass container is also not suitable for the reaction equipment.
Stability of NaCl
Sodium ion and chloride ion are stable in the water and do not involve in hydrolysis of water. So NaCl is a neutral salt.
Stability of NaOCl in water
Hypochlorite ion is not stable in water and involve in hydrolysis of water to produce HOCl and OH- ion. So aqueous solution will
basic due to release of hydroxyl ion. Due to formation of OH- ions, aqueous solution gets basic and show pH values of greater than 7.
But this solution is not basic as an aqueous NaOH solution.
Can I do the experiment of NaOH, Cl2 reaction in the laboratory?
Yes. You can. You can prepare cold NaOH solution by using ice crystals or cold water. But, you will be find difficult to find
chlorine gas in the laboratory. Then, you can try to make chlorine gas yourself. Mix dilute HCl solution and potassium
permanganate solution. That will give Cl2 gas. Feed produced Cl2 gas safely through tubes to the
NaOH solution.
reaction of chlorine with sodium hydroxide acid or base?
Reaction of chlorine with sodium hydroxide can be considered as an acid-base reaction. How do I say that?
First, chlorine dissolve in water and produce hydrochloric acid (HCl) and hypochlorous acid (HOCl). These two acids react with
NaOH separately to give NaCl and NaOCl respectively.
sodium hydroxide react with chlorine to form bleaching powder, equation for reaction
Sodium hydroxide is not used in the production of bleaching powder. Ca(OH)2 reacts with chlorine to produce bleaching powder.
Reaction of caustic and chlorine
Caustic soda is a another name for NaOH. So reactions are same as NaOH and Cl2.
caution pool chorine naoh?
You may feel, smell of chlorine gas on swimming pools. This happens due to formation of chlorine gas from NaOCl which is added as a disinfectant.
chlorine gas and hydroxide
In the presence of hydroxyl ions (OH-) ions, chlorine gas is reduced to Cl- and oxidized to
OCl- or ClO3-
ion. The oxidation product depends on the concentration and temperature of the hydroxide solution. If temperature is high,
chorine gas is oxidized to a its +5 oxidation state compound ClO3-.
Write a chemical equation for reaction between chlorine gas and dilute sodium hydroxide
NaOH + Cl2 → NaCl + NaClO + H2O
sodium and oxygen gas reaction?
If sodium piece is kept in the atmosphere, sodium readily reacts with oxygen and produce sodium oxide (Na2O).
But, in the presence of oxygen gas stream, sodium gives sodium peroxide as the product.
Sodium peroxide and sodium oxide
both reacts with water to produce NaOH.
Which gas is produced when NaOH is prepared?
Chlorine and hydrogen gases are formed when concentrated aqueous NaCl is electrolyzed to produce NaOH. To prevent the reaction of chlorine and NaOH, a membrane is placed between cathode and anode.
Does Sodium and chlorine gas reaction gives the same products as Sodium hydroxide and chlorine gas give?
Sodium hydroxide and chlorine gas reaction is completely different from
sodium and chlorine gas reaction.
2Na + Cl2 → 2NaCl
Chlorine and NaOH reaction have been included in following syllabuses.
- Cambridge International AS and A Level Chemistry
- General Certificate of Education (Advanced Level) GRADES 12-13
Related Tutorials to NaOH and Cl2



