N3- (Azide) Ion Lewis Structure | Steps of Drawing
Azide ion (N3-) has only 3 nitrogen atoms. In lewis structure of N3- ion contains two N=N bonds. Each outside nitrogen atoms have two lone pairs and center nitrogen atom does not have lone pairs. There are charges on all nitrogen atoms. Steps of drawing the lewis structure of N3- ion are explained in detail in this tutorial.
Lewis structure of N3- ion
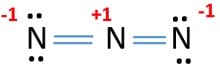
You can see there are -1 charges in both outside nitrogen atom and center nitrogen atom has +1 charge. Around center nitrogen atom, there are two sigma bonds and two pi bonds.
Steps of drawing the lewis structure of N3- ion
There are few steps to draw a lewis structure of a molecule or ion. Because N3- ion is an ion, all basic steps are used to draw it. So, you can learn good basic things of drawing lewis structures from this example.
- Find total number of electrons of the valance shells of nitrogen atoms
- Total electrons pairs as lone pairs and bonds
- Center atom selection
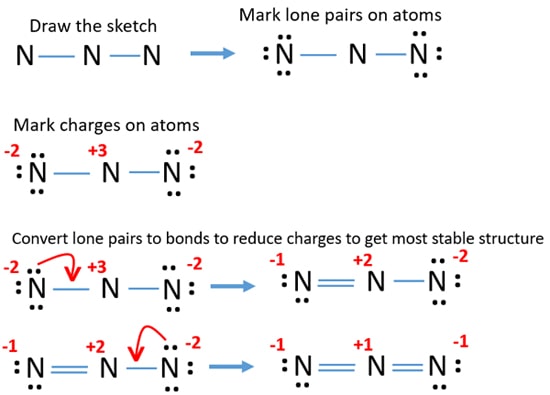
- Mark lone pairs on atoms
- Mark charges on atoms if there are charges on atoms.
- Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure.
Total number of electrons of the valance shells of N3- ion
Nitrogen is a group VA element in the periodic table and contains five electrons in its last shell.
To find out total valence electrons given by a particular element, you should multiply number of electrons of the valance shell by the number of atoms of that element in respective molecule.
- valence electrons given by nitrogen atoms = 5*3 = 15
Because there is a -1 charge in N3- ion, an extra electron is received to total valence electrons.
- electrons received due to -1 charge = 1
- Total valence electrons = 15 + = 16
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, N3- ion, total pairs of electrons are 8.
Center atom of N3- ion
There is no need to select center atom between atoms because there is only one element in N3- ion.

Lone pairs on atoms
Now, we knew the center atom and basic sketch of N3- ion. As the next step, we mark lone pairs on atoms. Remember that, there are total of 8 electron pairs as bonds and lone pairs.
- There are already 2 N-N bonds in the above drawn sketch. Now only 6 (8-2) electron pairs are remaining to mark on atoms.
- Usually, those remaining electron pairs should be started to mark on outside atoms (on nitrogen atoms). Each outside nitrogen atom will take 3 lone pairs and total of 6 electron pairs can be marked on all outside two nitrogen atoms.
- Now, all remaining lone pairs are marked and there is no lone pair to mark on center nitrogen atom (that is not a problem).

Mark charges on atoms and check the stability and minimize charges on atoms by converting lone pairs to bonds
After marking lone pairs on atoms, there are charges on every nitrogen atom and it is figured below.
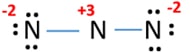
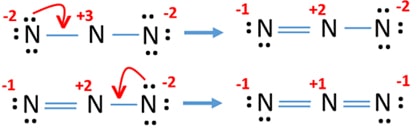
Having such charges on atoms is not good for stability of a molecule or ion. Therefore, we should try to reduce charges on atoms by converting lone pairs to bonds in possible occasions.
Questions
Are three a triple bond in N3- lewis structure
No, If we put a triple bond between two nitrogen atoms, we have to face a problem of one nitrogen atom having -2 charge and center nitrogen atom has +1 charge. That kind of charge distribution is not stable for a molecule or ion. Thereofore, having a triple bond between two nitrogen atoms is not possible in N3- lewis structure.