PCl5 (Phosphorus pentachloride) Lewis Structure
Phosphorus pentachloride (PCl5) contains five chlorine atoms and one phosphorus atom. In PCl5 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond (sigma bond). You can see there is no lone pairs on phosphorus atom in PCl5 as PCl3. In this tutorial, we will learn how to draw the lewis structure of PCl5 step by step with all theories.
PCl5 lewis structure
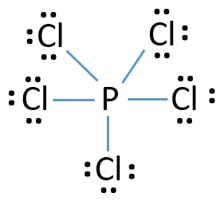
In this lewis structure of PCl5, center phosphorus atom has made five single bonds with five chlorine atoms. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. That means, there are ten electrons around phosphorus atom. This an example where some elements can keep more than eight electrons in their valence shell. Also, there are no charges on atoms in PCl5 lewis structure.
Steps of drawing PCl5 lewis structure
There are guidelines to draw a lewis structure properly and easily. Number of steps can be changed according the complexity of the molecule or ion. Now, we are going to study each step of drawing the lewis structure of PCl5 in next sections.
- Find total number of electrons of the valance shells of phosphorus and chlorine atom
- Total electrons pairs existing as lone pairs and bonds
- Center atom selection
- Mark lone pairs on atoms
- Mark charges on atoms if there are charges
- Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure.
Total number of electrons of the valance shells of PCl5
There are only two elements in phosphorus trichloride; phosphorus and chlorine. Phosphorus is a group VA element in the periodic table and has five electrons in its last shell (valence shell). Chlorine is a group VIIA element in the periodic table and contains seven electrons in its last shell.
- valence electrons given by phosphorus atom = 5 * 1 = 5
- valence electrons given by oxygen atoms = 7 * 5 = 35
- Total valence electrons = 5 + 35 = 40
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, PCl3, Total pairs of electrons are twenty (40/2) in their valence shells.
Selection of center atom of PCl3
When you draw a lewis structure, selecting the center atom is very important because structure of the molecule depend on the center atom selection. There are requirements to be the center atom. Having a high valence and being the most electropositive atom are the most important facts to be a center atom. In PCl5, there are only two elements to select the center atom. But, selecting the center atom is a challenging thing for PCl5. But, we are going to understand that now.
- having a high valence is important because center atom should have the ability to make lot of bonds around it.Phosphorus's maximum valence is 5 and chlorine's maximum valence is 7. From this fact, chlorine has the high priority to be the center atom.
- Phosphorus's and chlorine's electronegativity values are 2.1 and 3.0 respectively. in this case, phosphorus is more electropositive than chlorine. In this occasion, phosphorus has the highest chance to be the center atom.
- Now, this become confusing because we cannot decide what is the center atom from above two facts.
- Let's consider valence of phosphorus and chlorine atoms. We know, phosphorus has valence of 3 and 5. Chlorine has valence of 1,3,5 and 7.
- Because phosphorus's minimum valence is 3, there should be at least three bonds around phosphorus atom to fulfill the minimum valence. But, in chlorine, minimum valence is 1. Therefore, sometimes having one bond around chlorine atom is enough to make a covalent compound (Example HCl).
- From above 4 and 5 facts, we can decide phosphorus atom should be the center atom.
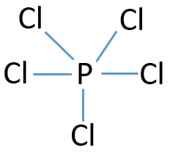
Mark lone pairs on atoms of PCl3
After determining the center atom and sketch of PCl5 molecule, we should start to mark lone pairs on atoms. Remember that, there are total of 20 electron pairs to mark as bonds and lone pairs in overall molecule.
- There are already 5 bonds in the above drawn sketch (5 P-Cl bonds). Now only fifteen (13-3=10) lone pairs are remaining to mark on atoms.
- First, those remaining electron pairs should be started to mark on outside atoms. Then, mark lone pairs on chlorine atoms. Each outside chlorine atom will take 3 lone pairs. Now, fifteen electron pairs were marked as lone pairs on outside 5 chlorine atoms. So, now there are no lone pairs to mark on rest of the atoms (on phosphorus atom).
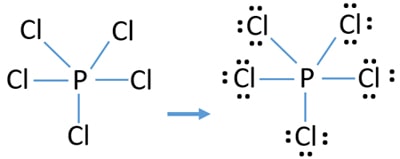
Mark charges on atoms and reduce charges to obtain most stable lewis structure
There are no charges on any of atoms. Therefore, we don't need to worry about reducing charges to get the most stable lewis structure. That means, we have already obtained the lewis structure of PCl5 after marking lone pairs on our sketch.
Questions