Sulfur dioxide and Water Reaction | SO2 + H2O
Sulfur dioxide (SO2) is averagely soluble in water and react with water to produce sulfurous acid (H2SO3). H2SO3 is a dibasic weak acid and partially dissociates to release H3O+ ions. We will discuss about those in detail in this tutorial.
We can write several equations to describe the generation of sulfurous acid and releasing H3O+ ion when sulfur dioxide gas is dissolved in water.
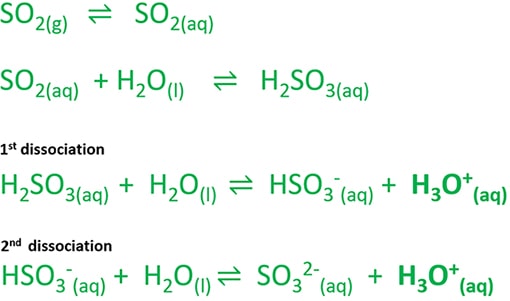
In this tutorial, we will discuss followings.
Solubility of sulfur dioxide in water
Sulfur dioxide gas is averagely soluble in water and we can write a reversible reaction as below.
SO2(g) ⇌ SO2(aq)
Reaction with water
Dissolved sulfur dioxide molecules reacts with water to produce sulfurous acid (H2SO3). This reaction is also a reversible reaction. Sulfurous acid is a weak dibasic acid and give very less quantity of H3O+ ions.
SO2(aq) + H2O(l) ⇌ H2SO3(aq)
Dissociation of sulfurous acid in water
Because H2SO3(aq) is a dibasic weak acid, there are two releasable H+ ions. Because of that we can write two reversible equations as below.
First dissociation of sulfurous acid
H2SO3(aq) + H2O(l) ⇌ HSO3-(aq) + H3O++
Second dissociation of sulfurous acid
HSO3-(aq) + H2O(l) ⇌ SO32-(aq) + H3O++
If specific amount of sulfur dioxide gas is fed into a closed system where material transfer is impossible, total system can be come to an equilibrium after some time if other physical parameters are kept constant.
Change of oxidation numbers
This reaction is not a redox reaction because oxidation numbers of atoms are not changed during the reaction process.
- In both sulfur dioxide molecule and sulfurous acid molecule, sulfur is at +4 oxidation state.
Physical and chemical observation when SO2 gas is dissolved
Here, we will see some physical observations and chemical properties changes during the reaction. These observations are important to identify compounds from other compounds in the qualitative analysis.
Colour changes
- There is no change in colour (colourless) when SO2 is dissolved and reacted with water. So H2SO3 is a colourless solution.
pH change of solution
Because sulfurous acid releases H3O+ ions, that solution should be acidic. So, pH value should be less than 7. However sulfurous acid is not strong as sulfuric acid considering acidic property.
Safety, health hazards and environmental impacts possible due to this reaction
- Sulfur dioxide is a toxic gas to humans.
- Considering environmental pollution, Because sulfurous acid has the capability of forming acid rain, emission of sulfur dioxide may cause to acid rains. So emissions of sulfur dioxide gases from industries and automobiles should be minimized to prevent acid rains.
Questions
How do I conduct this SO2 and H2O reaction in my laboratory?
Only challenge in this experiment is producing Sulfur dioxide gas. You can prepare Sulfur dioxide gas by adding dilute HCl acid to solid metal sulfite (such as sodium sulfite). Prepared Sulfur dioxide should be sent to water safely through a properly sealed pipeline to observe the reaction.
Which chemicals SO2 + H2O gives?
- Sulfurous acid
- Bisulfite ion
- Sulfuric acid
- Hydrogen sulfide
Only Sulfurous acid and Bisulfite ion are given as products when SO2 dissolve and react with water.
sulfur dioxide + water = sulfurous acid, is this correct?
This reaction is reversible, as sulfurous acid is formed, back reaction takes place to form Sulfur dioxide gas again.
What is correct about the reaction of sulfur dioxide and water?
- It gives a strong acidic solution.
- It gives a yellow colour solution.
- Sulfurous acid is generated when Sulfur dioxide gas dissolve in water.
- Sulfur dioxide is highly soluble in water and form an acidic solution.
Answer:
Sulfur dioxide is averagely soluble in water and give a weak acid (Sulfurous acid) as the result.
- Choice 1 is incorrect.
- Aqueous sulfurous acid solution is colourless. So, choice 2 is incorrect.
- Choice 3 is correct.
- Sulfur dioxide is not highly soluble in water. So, choice 4 is also incorrect.
Is SO2 + H2O reaction is similar to SO2 + H2O reaction?
Solubility of Sulfur trioxide (SO3) is more poor compared to Sulfur dioxide. Therefore, a tiny amount of Sulfur trioxide dissolve in water and little amount of them react with water to form Sulfuric acid.