1,2-dibromopropane (C3H6Br2) Lewis Structure
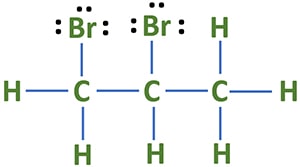
1,2-dibromopropane (C3H6Br2) is an alkyl halide compound which has two bromine atoms. As IUPAC name suggests, we can get a clear understanding of the structure of C3H6Br2 molecule. In lewis structure of 1,2-dibromopropane has only single bonds. There are three lone pairs on each bromine atom.
Explanation of Lewis structure of 1,2-dibromopropane (C3H6Br2)
Number of atoms in Lewis structure of 1,2-dibromopropane
- There are three carbon atoms, two bromine atoms and six hydrogen atoms in the molecule.
- As IUPAC name suggest (1,2-dibromo), there are two bromine atoms
Sigma and Pi bonds around carbon, hydrogen and bromine atoms 1,2-dibromopropane
- There are only sigma bonds around all atoms. There are four sigma bonds around one carbon atom.
- No double and triple bonds around carbon atoms.
Lone pairs around carbon, hydrogen and bromine atoms 1,2-dibromopropane
- There are no lone pairs around carbon atoms.
- Each Bromine atom has three lone pairs.
- Because Hydrogen atom has made a single bond with Carbon atom, Hydrogen atom cannot keep more lone pairs.
Questions
Alkene (Ethene, Propene)
and bromine reaction
1-bromo-2-chloropropane (C3H6BrCl) lewis structure
phosphorus oxychloride (POCl3) lewis structure
BrO2- lewis-structure
B2O3
lewis structure
Propene + HBr reaction
Alkene hydration
- Ethene + dilute H2SO4
Alkane chlorination - Methane and Cl2 reaction
Clemmensen reduction of aldehydes and ketones
Hofmann degradation of amides
Aldol condensation of aldehydes and ketones