NH4+ (Ammonium ion) Lewis Structure
In NH4+ (ammonium ion) lewis structure, there are four sigma bonds around nitrogen atom. there is a +1 charge on nitrogen atom. Steps of drawing the lewis structure of NH4+ are explained in this tutorial.
NH4+ lewis structure
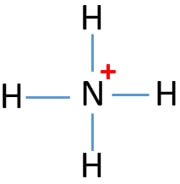
You see, there are four hydrogen atoms around nitrogen atom. Therefore, nitrogen atom is the center atom. Also, there is a +1 charge on nitrogen atom.
Steps of drawing lewis structure of NH4+
There are several steps to draw the lewis structure of NH4+. But, These steps are explained in detail in this tutorial.
- Find total number of electrons of the valance shells of hydrogen atoms and nitrogen atom
- Total electrons pairs as lone pairs and bonds
- Center atom selection
- Mark lone pairs on atoms
- Mark charges on atoms if there are charges on atoms.
- Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure.
Total number of electrons of the valance shells of NH4+
There are two elements in NH4+; hydrogen and nitrogen. Also, you have to consider there is a +1 charge in ammonium ion. Hydrogen is a group IA element and has only one electron in its last shell (valence shell). Nitrogen is a group VA element in the periodic table and contains five electrons in its last shell. Now we know how many electrons are includes in valence shells of hydrogen and nitrogen atoms.
To find out total valence electrons given by a particular element, you should multiply number of electrons of the valance shell by the number of atoms of that element.
- valence electrons given by hydrogen atoms = 1 * 4 = 4
- valence electrons given by nitrogen atom = 5*1 = 5
- Because there is a +1 in NH4+, an electron should be reduced from the summation of total valence electrons.
- Total valence electrons = 4 + 5 - 1 = 8
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, NH4+, Total pairs of electrons are 4.
Center atom of NH4+ molecule
To be the center atom, ability of having greater valance is important. Then, from hydrogen and nitrogen atoms, which atom has the highest valence? Maximum valence of nitrogen is five. Hydrogen's only valence is one. Therefore, nitrogen atom should be the center atom of NH4+. Now, we can draw the sketch of NH4+ to describe how atoms are attached with each other.
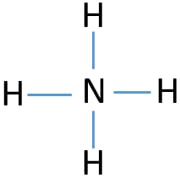
Mark lone pairs on atoms
After determining the center atom and sketch of NH4+ ion, we can start to mark lone pairs on atoms. Remember that, there are total of four electron pairs.
- There are already four N-H bonds in the above drawn sketch. Now zero (4-4) electron pair remains to mark on atoms.
Mark charges on atoms
There is a +1 charge on nitrogen atom and no charges on hydrogen atoms.
Check the stability and minimize charges on atoms by converting lone pairs to bonds
Because there are no lone pairs to convert to bonds, we cannot reduce charges furthermore. Also, in current structure has a charge only on one atom. Therfore, we don't need to worry about reducing charges of atoms.
Shape and Geometry of ammonium ion
There are four sigma bonds around the nitrogen atom. Therefore, shape of NH4+ is tetrahedral.
Summation of number of sigma bonds and lone-pairs around nitrogen atom is four. Therefore geometry should be tetrahedral.
Questions
Can I draw the NH4+ lewis structure from NH3 lewis structure?
First, we need the reaction of forming of NH4+ ion from NH3. NH4+ ion is made from NH3 and H+ ion. The lone pair in NH3 is given to the H+ ion to make a new N-H bond. Now, there are four N-H bonds around nitrogen atom.
See the reaction of ammonia and HCl.
Lewis structure of NH4+ has a +1 charge on nitrogen atom. Therefore is it a stable structure?
First, we should know, atoms in a lewis structure can contain charges. When spread of charges around the ion or molecule is low, that structure become more stable. But, when there are charges on lot of atoms and cannot reduce charges furthermore, that structure is very unstable. But, when there are very less number of charges on atoms, that structure becomes the most stable lewis structure.
What will happen to positive charge of nitrogen atom in NH4+
Nitrogen is an element which has high electronegativity. Whrn nitrogen has a +1 charge in NH4+ ion, that nitrogen atom attracks electrons of bonds towards nitrogen atom to reduce +1 charge.
Can I say ammonium ion has acidic characteristics by looking NH4+ Lewis structure?
In ammonium ion, you cannot see a negative charge or lone pairs on atoms. Therefore, NH4+ cannot show basic characteristics. But, because there is a +1 charge on nitrogen atom. Because of that and nitrogen's high electronegativity, hydrogen atoms are positively charged. Therefore, basic compounds can attack those hydrogen atoms. Otherwise, we can say ammonia can release those hydrogen atoms as H+ ions to show acidic characteristics.
How do you draw NH4+ Lewis structure from NH3 Lewis structure?
Ammonia shows basic characteristics due to the presence of a lone pair on nitrogen atom. Therefore, it can attack H+ ions to make a bond. Like that, a new bond is former between nitrogen and hydrogen atom.