Chemical Bonds, Primary and Secondary Bonds with Properties
There are 118 of naturally occurring, known elements. Most of them do not exist as single elements. Some elements exist as diatomic molecules consisting of two atoms of the same element. Also, some other elements exist as chemical compounds. Chemical compounds consist of two or more elements which are connected by different kinds of chemical bonds.
Written by: , Sandeepani Wijekoon(undergraduate), Department of Chemical and Process Engineering, University of Peradeniya, Sri Lanka
Why do elements not exist as the way they are?
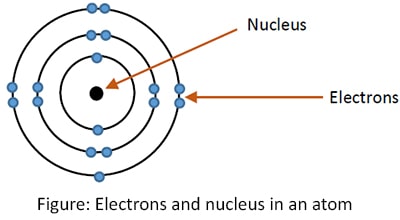
As we know, the smallest unit of the matter is an atom. An atom consists of a nucleus, electrons, protons and neutrons. Protons and neutrons are contained in nucleus. Electrons are in atomic orbitals. The distribution of the electrons in these orbitals is called electronic configuration.
The electronic configuration of an atom effects to its stability. An electronic configuration is said to be stable, if the valence shell (outermost shell) contains its maximum number of electrons; either two or eight. Only first shell contains maximum of two and every other shell can contain maximum of eight electrons. If this condition is not achieved an atom is said to be unstable. If it doesn't have a stable electronic configuration atoms cannot exist in nature. That's why atoms do make bonds! Atoms make different kind of bonds to obtain its stability.
Hence, a chemical bond is an attractive force between atoms, molecules or ions resulted by the rearrangement of its electrons to obtain its stable electronic configuration.
Types of bonds
There are two main types of bonds
.- Primary bonds
- Secondary bonds
Primary bonds
Primary bonds are divided into three types.
- Ionic bonds
- Covalent bonds
- Metallic bonds
Ionic bonds examples and properties
Let's see how an ionic bond forms by an example.
Sodium chloride (NaCl) as a ionic bond
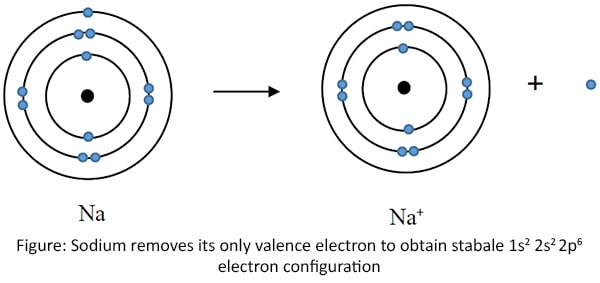
The electron configuration of Sodium (Na) atom is 1s2 2s2 2p6 3s1 (also electron configuration 2, 8, 1).
As mentioned in the previous section, an atom should have maximum number of electrons in the valence shell to be stable. Since sodium atom has
only one electron in its valence shell, it should receive seven more electrons or it should remove that electron to get the stability. Receiving seven electrons is
impossible, because it requires a massive energy. Hence, sodium removes its only valence electron from outermost shell.
In an atom, always number of protons in the nucleus and number of electrons in the orbitals are equal. Since protons are positively charged and electrons are negatively charged, any atom is neutral before any kind of chemical reaction.
When Na removes one electron, it obtains a positive charge.
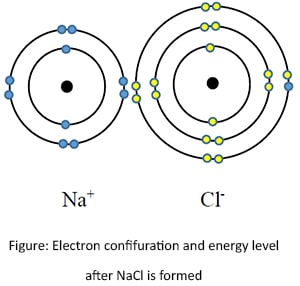
Let's consider a Chlorine (Cl) atom which has the electronic configuration of 2, 8, 7. To gain the stability, it is much easy to receive an electron. Therefore Cl receives an electron and obtain a negative charge.

Now let's consider how Sodium chloride forms. Since Na+ and Cl- have equal and opposite charges, they are getting bonded very strongly together by electrostatic force between them.
Magnesium chloride (NaCl) as a ionic bond
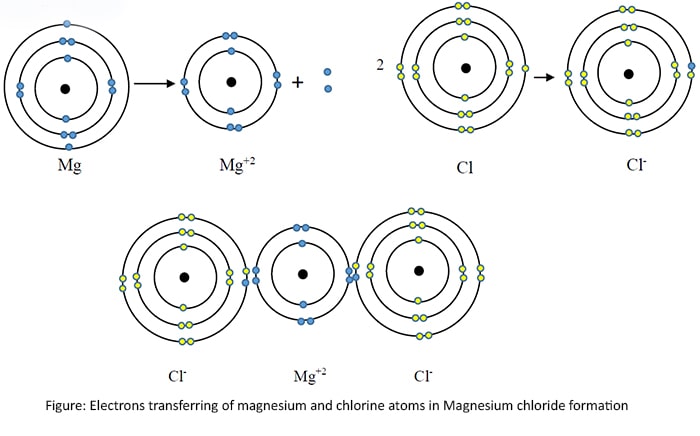
Therefore an ionic bond is a strong electrostatic attractive force between oppositely charged ions.
Covalent bonds
Covalent bond is formed by sharing electrons between the atoms which are being bonded to acquire the stable electronic configuration. Bonds are formed by all of the atoms which contribute to form the bond. Covalent bonds can be formed by two or more electrons.
- Diatomic molecules are formed by sharing electrons between same atoms. Examples: H2, O2, N2
- Hetero atomic molecules are formed by sharing electrons between different atoms. Examples: H2O, CO2, NH3
Let's look at some examples.
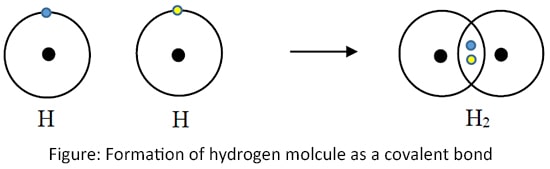
Hydrogen gas (H2)
Hydrogen gas is formed by sharing two electrons, one from each Hydrogen atom. It is called 'Single bond'. (Only the outermost shells are shown here)
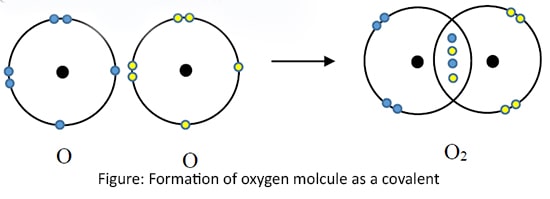
Oxygen gas (O2)
O2 is formed by sharing four electrons, two from each Oxygen atom. This bond is called 'Double bond'. (Only the outermost shells are shown here)
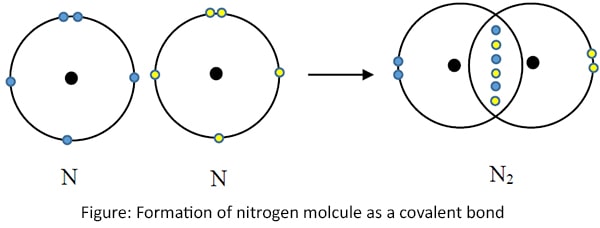
Nitrogen gas (N2)
Nitrogen gas is formed by sharing six electrons, three from each Nitrogen atoms. This type of bond is called 'Triple bond'. (Only the outermost shells are shown here)
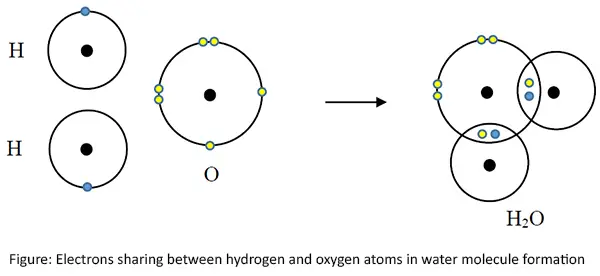
Covalent bonds in water molecule (H2O)
Since every atom contributing to form the bond, need to obtain its stable electronic configuration, electrons are shared according to the requirement. Oxygen atom forms two single bonds with each hydrogen atom sharing one electrons with each in water molecule. (Only the outermost shells are shown here.)
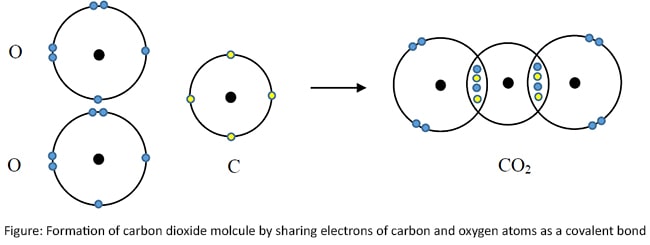
Covalent bonds in carbon dioxide (CO2)
There are covalent bonds between carbon and oxygen atoms in carbon dioxide molecule.
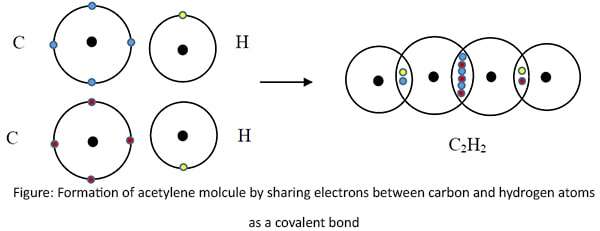
Covalent bonds in acetylene molecule
There are covalent bonds between carbon-carbon and carbpn-hydrogen atoms in acetylene.
Metallic bonds
All metals exist as metallic lattices. The valence electrons of metals are weakly attached to the nucleus. Hence, they are able to escape the nucleus and atom remains positively charged ion. Those electrons freely move in the lattice. This electron distribution leads to a negatively charged electron cloud in the lattice. The attraction between the positive ion core and the electron cloud forms the metallic bond.
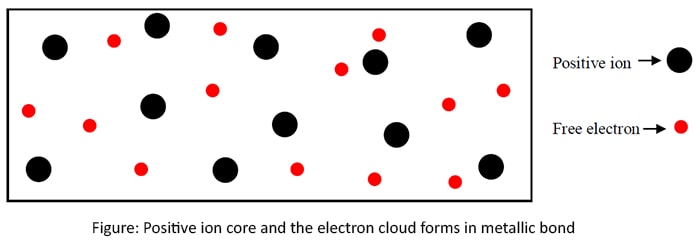
The behavior of the free electrons causes high thermal conductivity and high electrical conductivity. The number of escaped electrons and the metallic radius cause the strength of the metallic bond.
Secondary bonds
Secondary bonds are formed due to the intermolecular attraction where valence electrons neither shared nor donated. Intermolecular attraction are occurred due to the dipoles formed by uneven distribution of charges.
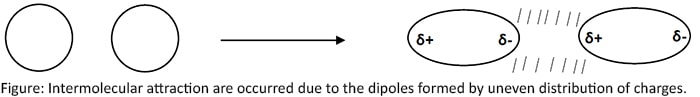
There are three main types of secondary bonds.
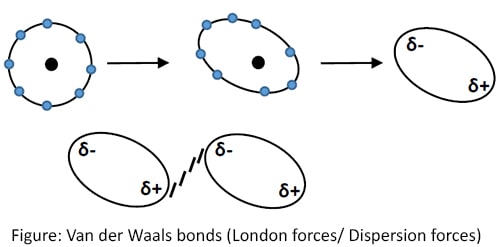
Van der Waals bonds (London forces/ Dispersion forces)
The attraction force between molecules due to self-induced temporary diploes is called Van der Waals bond. Due to uneven electron distribution molecule get slightly polarized. Thus a weak bond is formed.
These bonds hold molecules together in organic substances to form solids. Since Van der Waals bonds are weak, melting point of organic substances are often low.
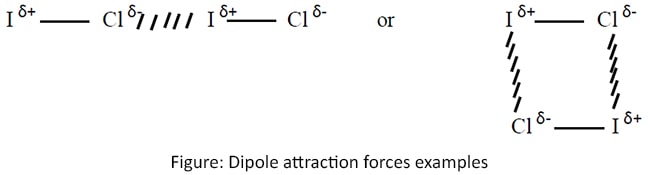
Dipole attraction forces
Attraction forces among different polar molecules which have permanent dipoles. The partially positive end attract to the partially negative end of the other molecule.
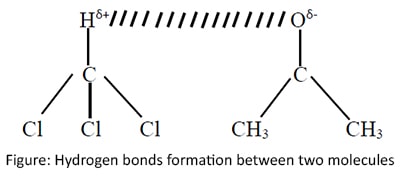
Hydrogen bonds
Hydrogen bond is a special type of dipole attraction forces. These bonds are formed when a Hydrogen atom and a high electronegative atom is present in the dipole.
Either Nitrogen or Oxygen or Fluorine atom must be present to form a hydrogen bond.
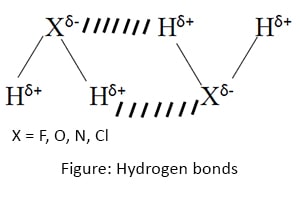
Also sometimes Hydrogen bonds may form when there are no X-H bonds in one molecule, but when two molecules have Hydrogen atom in one molecule and X atom in the other.