Lewis Structure of ClO4- (Perchlorate ion)
Lewis structure of ClO4- ion is drawn step by step in this tutorial. Total valence electrons of given by four oxygen atoms,chlorine atom and negative charge are considered to draw the ClO4- lewis structure.
Lewis Structure of perchlorate ion
There are four oxygen atoms and chlorine atom in perchlorate ion.
Now, we are going to learn, how to draw the lewis structure of ClO4- ion step by step. You will learn all steps and rules of lewis structure drawing.
Steps of drawing ClO4- lewis structure
Following steps are required to draw ClO3- lewis structure and they are explained in detail in this tutorial.
- How to find total number of electrons of the valance shells of chlorine and oxygen atoms and including charge of the anion
- How many electrons pairs in valence shells
- Determine center atom from chlorine and oxygen atom
- Put lone pairs on atoms
- Stability of lewis structure - Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain the best lewis structure.
Drawing correct lewis structure is important to draw resonance structures of ClO4- ion.
Total number of electrons of the valance shells of chlorine and oxygen atoms and charge of the anion
There are one chlorine atom and four oxygen atoms in the chlorate ion. Also there is a -1 overall charge on the ClO3- ion.
Chlorine and oxygen are located at 7 and 6 groups respectively in the periodic table. So chlorine has seven electrons in its valence shell and for oxygen atom, there are six electrons in its valence shell.
- Total valence electrons given by chlorine atoms = 7*1 = 7
There are three oxygen atoms in ClO4- ion, Therefore
- Total valence electrons given by oxygen atoms = 6 * 4 = 24
Due to -1 charge, another electrons is added
- Due to -1 charge, received electrons to valence electrons= 1
- Total valence electrons = 7 + 24 + 1 = 32
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, ClO4- there are 32 valence electrons, so total pairs of electrons are 16. In next steps, we are going to mark those 16 lone pairs on oxygen atoms and chlorine atoms as bonds and lone pairs.
Center atom of ClO4- ion
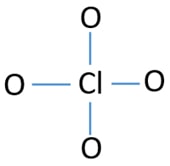
To be the center atom, ability of having greater valance is important. Chlorine can show valence 7. But, oxygen's maximum valence is 2. Therefore chlorine has more chance to be the center atom (See the figure). So, now we can build a sketch of ClO4- ion.
Sketch of ClO4- ion
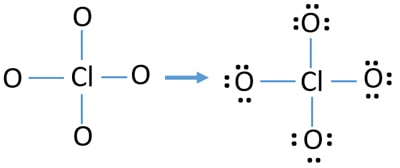
Lone pairs on atoms
- There are already four Cl-O bonds in the sketch. Therefore only twelve valence electrons pairs are remaining to draw the rest of ion.
- Next step is, marking those ten valence electrons pairs on outside atoms (in this case, oxygen atoms) as lone pairs. One oxygen atom will take three lone pairs following the octal rule (oxygen atoms cannot keep more than eight electrons in their valence shells). Therefore, twelve electrons pairs are marked on three oxygen atoms. Now, all electrons pairs are finished due to marking on oxygen atoms.
- So, there is no valence electrons pair to mark on chlorine atom.
Check the stability of drawn structure of ClO4- ion and reduce charges on atoms by converting lone pairs to bonds
Check charges on atoms and mark them as below. Charges are important to decide the best lewis structure of the ion because in the best lewis structure, charges should be minimized.

The drawn structure for ClO4- is not a stable structure because oxygen atoms and chlorine atoms have charges. Also, when charge of an atom (in chlorine atom, there is a +3 charge) is large, that structure become more unstable and cannot be a good lewis structure. When a molecule or ion has so many charges on atoms, that structure is not stable. Now, we should try to find a more stable structure.
Now, we should try to minimize charges by converting lone pair or pairs which exist on oxygen atoms to bonds. So we convert one lone pair of one oxygen atom as a Cl-O bond as in the following figure.
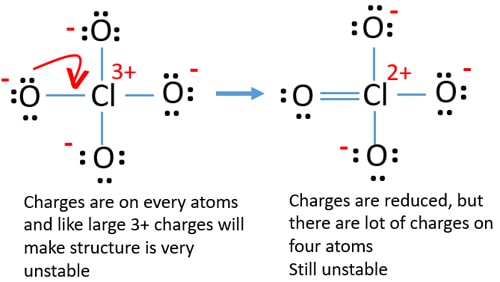
Now there is a double bond between chlorine and one oxygen atom. There are also three single bonds (Cl-O) with chlorine atom and other two oxygen atoms.
But, there are still charges on atoms and given structure is not stable yet. If possible, we should reduce charges furthermore. Another lone pair on another oxygen atom is transferred as a Cl-O bond.
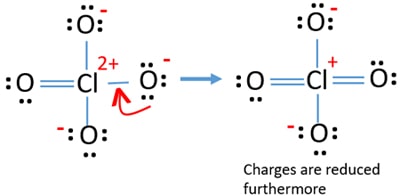
Now, there are twelve electrons around chlorine atom. This is acceptable because chlorine can keep more than eight electrons chlorine has unfilled 3d orbits.
In new structure, charges of atoms are reduced furthermore. But, we can convert one more lone pair on another oxygen atom to make an another double bond. Now you understand this structure of ClO4- is more stable than previous structure due to less charges on atoms.
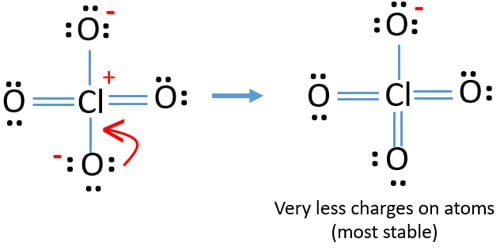
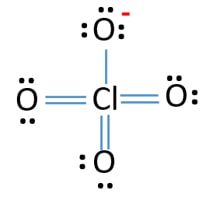
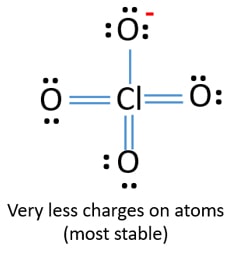
ClO4- Lewis structure
Questions asked by students
Ask your question and find the answer free.how many lone pairs are found in the lewis structure for the perchlorate ion, ClO4--?
In last shells, there are nine lone pairs on atoms. There are three oxygen atoms which are connected through double bonds to chlorine atom. Each of those oxygen atoms have two lone pairs. Remaining oxygen atom has three lone pairs and ceenter atom, chlorine does not has lone pairs.
What is the charge of ClO4-
Charge of ClO4- ion is -1. This -1 negative charge is located at an oxygen atom.
ClO4-lewis structure molecular geometry
Around chlorine atom, there are four σ bonds zero lone pairs. Therefore shape of the anion around chlorine atom is tetrahedral.