Methanol to Acetic Acid (Ethanoic acid)| CH3OH to CH3COOH Conversion
Methanol is an alcohol compound and acetic acid is a carboxylic acid compound. Both are widely used in chemical industry. Methanol (CH3OH) can be converted to acetic acid (CH3COOH) by different methods and they are explained in detail in this tutorial.
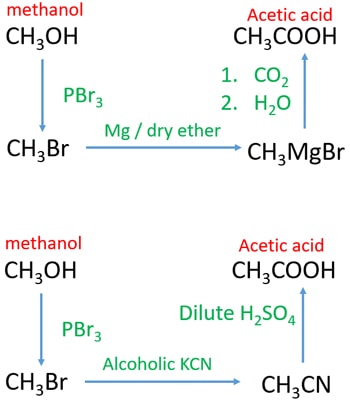
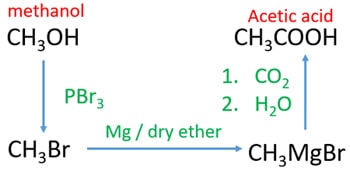
Methanol to acetic acid through grignard reagent
- Add PBr3 to methanol. You will get bromomethane which is a gaseous compound at room temperature.
- Send bromomethane through dry ether and magnesium. It will give Methyl magnesium bromide (grignard reagent).
- Send carbon dioxide gas through prepared Methyl magnesium bromide. After that add water or dilute sulfuric acid to get acetic acid (ethanoic acid)
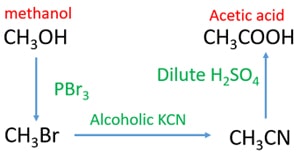
Methanol to acetic acid through Acetonitrile
- Add PBr3 to methanol. You will get bromomethane which is a gaseous compound at room temperature.
- Send bromoethane through through alcoholic KCN. It will give acetonitrile (Methyl cyanide).
- Finally, add water or dilute sulfuric acid to hydrolyze acetonitrile. It will give acetic acid.
Related tutorials
Oxidation of
alcohols
2-propanol to propanol
Oxidation of propanol
carbonyl compounds Aldehyde, Ketone
Propanol to propanone