Lewis Structure of Hexane (C6H14) (n-Hexane)
Hexane (C6H14) is an alkane compound which has six carbon atoms and fourteen hydrogen atoms. In lewis structure of C6H14, there are five single C-C bonds and fourteen C-H bonds. Hexane is widely used as a non-polar solvent in analytical laboratories and you will understand why hexane become a non-polar solvent after studying the lewis structure of Hexane.
Lewis structure of C6H14
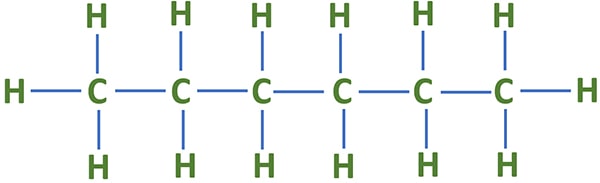
Now, we are going to see some specifications of lewis structure of C6H14 molecule.
Number of atoms in Lewis structure of C6H14
- There are six carbon atoms and fourteen hydrogen atoms in the molecule.
- As name suggest n-hexane, there is a linear carbon chain in n-hexane. So there are no sub-carbon chains except the main carbon chain.
Characteristics of lewis structure of C6H14
Here, we see followings of C6H14 molecule.
- Sigma and Pi bonds around carbon and hydrogen atoms in Hexane lewis structure
- Lone pairs around carbon and hydrogen atoms
- Polarity and use of as a non-polar solvent
Sigma and Pi bonds around carbon and hydrogen atoms in Hexane lewis structure
- There are only sigma bonds around all atoms. There are total of nineteen sigma bonds in the molecule. Among those nineteen sigma bonds, there are fourteen C-H sigma bonds and five C-C bonds. SO each carbon has made four sigma bonds.
- No double and triple bonds around any atom.
Lone pairs around carbon and hydrogen atoms
- There are no lone pairs around carbon atoms.
- Because Hydrogen atom has made a single bond with Carbon atom, Hydrogen atom cannot keep more lone pairs.
Polarity and use of as a non-polar solvent
Hexane is used as a common non-polar solvent in chemistry. Because, polarity of C-H bond is very less due to small electronegativity difference between carbon and hydrogen atoms. Therefore, there are only weak Vanda Vaal Forces between hexane molecules. But due to considerable molar mass, hexane exist as a liquid at room temperature.
Questions
Are there any double bond in hexane lewis structure?
Hexane is an alkane compound with six carbon atoms. There are only single bonds between carbon atoms in alkane compounds and no any double bonds.
Are there lone pairs on carbon atoms in hexane lewis structure?
There should be eight electrons around the carbon atom to form a stable molecule. In hexane, there are four C-H bonds around every Carbon atom.