Calcium Carbonate and Hydrochloric Acid Reaction | CaCO3 + HCl
Calcium carbonate (CaCO3)
is a metal carbonate compound and reacts with hydrochloric acid (HCl) to
produce carbon dioxide (CO2), calcium chloride (CaCl2) and water. You can see, carbon
dioxide gas is released through the solution. Specifically, though Calcium carbonate is not soluble in water, it readily dissolves
in dilute Hydrochloric acid solution.
Content
- Products of Calcium carbonate (CaCO3) and Hydrochloric acid (HCl) reaction
- Reaction of metal carbonate compound and a dilute acid
- Physical states of given products
- Balanced chemical equation of CaCO3 and HCl reaction with physical states
- Properties and characteristics of CaCO3 and HCl acid reaction
- Acidity of the aqueous solution
- Exothermic reaction
- Calculate purity of CaCO3 by HCl acid with an example
- Example calculation: Calculate purity of CaCO3 which is contaminated with inert substances.
Products of Calcium carbonate (CaCO3) and Hydrochloric acid (HCl) reaction
Metal carbonate compounds react with dilute acids and emit carbon dioxide gas.
As, all other metal carbonate compounds, Calcium carbonate reacts with dilute acids such as HCl.
- Calcium chloride, carbon dioxide gas and
water will be given as products during
this reaction.
- Because reaction is being carried in an aqueous phase, produced Calcium chloride dissolve in the aqueous solution. Some carbon
gas quantity can be dissolved in the aqueous solution. If reaction is carried out in a open container to atmosphere, all formed
carbon dioxide gas can be removed to atmosphere with time.
CaCO3 + HCl → CaCl2 + CO2 + H2O
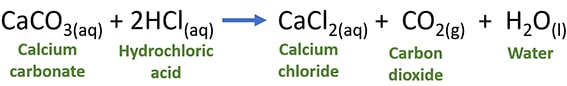
Balanced chemical equation of CaCO3 and HCl reaction with physical states
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Calcium carbonate is not soluble
in water and exists as white precipitate in the water. When aqueous hydrochloric acid is added, you can observe air bubbles are generated and
calcium chloride, carbon dioxide and water are formed.
- Calcium chloride (CaCl2) is soluble in water and colorless. So,
it exists as an aqueous solution. Therefore,
you can see, white precipitate is dissolved and colorless solution is formed with time.
- Also, due to formation of carbon dioxide gas, gas bubbles are rising to the top of the solution.
Properties and characteristics of CaCO3 and HCl acid reaction
Here, we will see how some physical and chemical properties vary with the time when reaction is carried out.
Acidity of the aqueous solution
When calcium carbonate is precipitated in water, that solution become weak basic due to the presence of
partial dissociation of carbonate
ions. When aqueous HCl is added to the solution where Calcium carbonate precipitate is,
carbonate ion is
converted to the carbon dioxide gas and alkalinity of the aqueous solution decreases.
Exothermic reaction
When Calcium carbonate reacts with hydrochloric acid, heat is released to the environment. Therefor this reaction is an exothermic reaction.
Calculate purity of CaCO3 by HCl acid
This reaction is used to calculate purity of CaCO3 samples when they are mixed with impurities.
If impurity material does not react with dilute hydrochloric acid, You can conduct this experiment. Released carbon dioxide
volume is measured and then calculate the released amount (mol) of carbon dioxide gas. Then, you can calculate the reacted
calcium carbonate amount and mass.
Example calculation: Calculate purity of CaCO3 which is contaminated with inert substances.
There is a solid mixture which include Calcium carbonate and has other inert stuff. 5.5 g of that mixture is mixed with 10 cm3 of
1.0 mol dm-3 HCl solution. After allowing reaction to be completed, pH value
of the aqueous solution is measured and pH value was 3. Calculate the composition of Calcium carbonate in weight basis (m/m%).
Assumption you need to make during the calculation
- Final volume of the solution after reaction is completed is as 100 cm3 as the initial volume.
Calculate final / remaining HCl concentration (mol dm-3)
Use the pH = -log10[H+] equation.
- pH of remaining solution = 3
- pH = -log10[H+]
- 3 = -log10[H+]
- H+ = 1 * 10-3 mol dm-3
Calculate final / remaining HCl amount (mol)
Use the C = n/V equation.
- nremaining HCl amount = 1 * 10-3 mol dm-3 * 100 * 10-3 dm3
- nremaining HCl amount = 0.0001 mol
Calculate initial HCl amount (mol) before adding to the solid mixture
Use the C = n/V equation.
- Initial HCl amount = 1 mol dm-3 * 100 * 10-3 dm3
- Initial HCl amount = 0.1 mol
Calculate reacted / consumed HCl amount (mol) during the reaction
Reacted/ consumed HCl amount = Initial HCl amount - remaining HCl amount
- Reacted / consumed HCl amount = 0.1 mol - 0.0001 mol
- Reacted / consumed HCl amount = 0.0999 mol
Calculate reacted / consumed CaCO3 amount (mol) during the reaction
Use the stoichiometric balanced equation
- Reacted / consumed CaCO3 amount = 0.0999 mol / 2
- Reacted / consumed CaCO2 amount= 0.04995 mol
Calculate reacted / consumed CaCO3 mass (g) during the reaction
Use the n = m/M equation
- m - nM
- Reacted / consumed CaCO3 mass = 0.04995 mol * 100
- Reacted / consumed CaCO3 mass = 4.995 g
Calculate mass composition of CaCO3 mass (g) in the solid mixture
- Mass composition of CaCO3 mass (g) in the solid mixture = 4.995 * 100 / 5.5000
- Mass composition of CaCO3 mass (g) in the solid mixture = 90.81%
Questions asked by students
Ask your question and find the
answer free.
When HCl reacts with calcium carbonate why heat is released?
If heat is released from a reaction, it means products of the reactions are more stable than reactants. When something is stable, it should have less energy. When HCl reacts with calcium carbonate, calcium chloride and carbon dioxide gas are given.
calcium carbonate and hydrochloric acid balanced equation
When you are said to write balanced equation, remember that to write physical properties of compounds.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
What happens when CaCO3 reacts with dilute HCl acid?
Calcium carbonate reacts with dilute acids to produce a calcium salt, water and carbon dioxide gas: calcium carbonate +
hydrochloric acid → calcium chloride + water + carbon dioxide.
What does HCl and CaCO3 produce?
Calcium chloride, carbon dioxide and water are given as products.
Can I identify calcium carbonate from zinc carbonate from this reaction?
Both carbonates are not insoluble in water and form white precipitates. Calcium carbonate and zinc carbonate reacts with
dilute hydrochloric acid and emit carbon dioxide gas form colorless solutions. (CaCl2 and ZnCl2 are
colorless solutions).
But, treating hydrogen sulfide gas to calcium chloride and zinc chloride can be used to identify solutions. Calcium sulfide
is soluble in water. But, ZnS is not
a soluble sulfide in water and form a white precipitate.
Related Tutorials
Sodium carbonate and HCl reaction
FeCl2 + NaOH reaction
NaOH and chlorine gas reaction
Solubility
of metal carbonate compounds
CO2 lewis structure
