Grignard reagent reaction with alcohol | Ethanol + Grignard
Grignard reagent and alcohol reaction give a hydrocarbon as a product which is an alkane compound in most occasions. Alkyl group of Grignard reagents are strong alkalis and nucleophiles. Grignard reagent behaves as a nucleophile and get the hydrogen atom from the alcohol group.
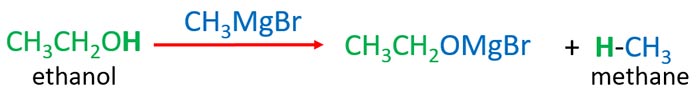
Methyl magnesium bromide and ethanol reaction
Methyl magnesium bromide reacts with ethanol to give methane. Methyl group in the grignard reagent has a lone pair with a negatice charge. This methyl group attacks the hydrogen atom of -OH group and takes that hydrogen atom towards methyl group. It forms methane.
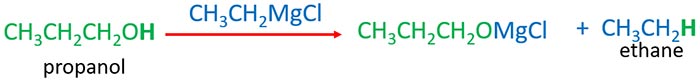
Grignard reagent and propanol reaction
Ethyl magnesium chloride reacts with propanol to give ethane.
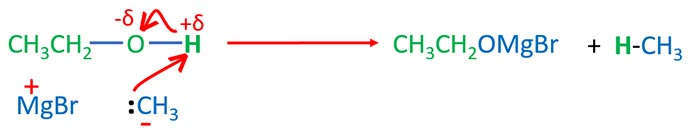
Grignard reagent and alcohol reaction mechanism
Grignard reagents are strong nucleophiles which can attach positively charged parts. The hydrogen atom in the -OH part of the alcohol is attacked by grignard reagent. Then O-H bond is braked and hydrogen atom connects to the alkyl group of grignard reagent.
Questions
which of the following can form methane gas with methyl magnesium bromide?
- CH3CH3
- CH3Br
- H2O
Methyl magnesium bromide reacts easily with water to give methane.
Methyl magnesium bromide reacts with CH3Br and gives ethane. But methyl magnesium bromide does not react with CH3CH3.