Ethylbenzene to benzoic acid and benzene
Ethylbenzene to benzene organic conversion is a two step process. First ethylbenzene reacts with acidic potassium permanganate to give benzoic acid. Then benzoic acid reacts with soda lime (decarboxylation) to give benzene.
Toluene (methylbenzene) can be used to prepare benzene.
Ethylbenzene to benzoic acid
Ethylbenzene reacts with acidic potassium permanganate and give benzoic acid which is a white solid. Benzoic acid is a carboxylic acid compound.
Instead of acidic potassium permaganate, acidic potassium dichromate and acidic potassium chromate can be used as reagents.
- acidic potassium permaganate - H+ / MnO42-
- acidic potassium dichromate - H+ / Cr2O72-
- acidic potassium chromate - H+ / CrO42-
Benzoic acid to benzene
Soda lime (NaOH / CaO) reacts with benzoic acid to give benzene. Carboxylic group is removed from benzoic molecule to give benzene. This reaction is called as decarboxylation. As benzoic acid to benzene, other carboxylic acids can be converted to hydrocarbons from decarboxylation.
Decarboxylation of carboxylic acids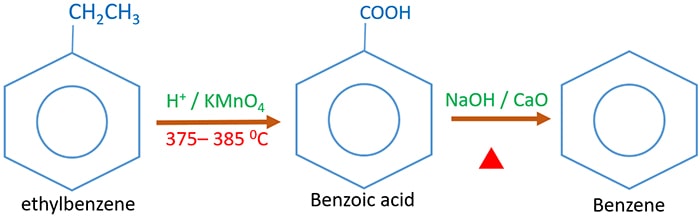
Alkyl benzene to benzoic acid
Alkyl benzene compounds can be oxidized to benzoic acid using strong oxidize reagents. At least one carbon atom should be attached to the benzene ring to prepare benzoic acid.
Benzaldehyde is oxidized to benzoic acid by strong oxidize reagents.
Toluene to benzoic acid
As ethylbenzene, toluene also can be used to prepare benzoic acid.
Benzoic acid and benzene properties
Benzoic acid and benzene are toxic compounds.
Benzoic acid reacts with Na, NaOH, NaHCO3, Na2CO3. Therefore benzoic acid show acidic characteristic. But benzene does not react with sodium.
Benzoic acid is a white precipitate and does not dissolve in water.
Benzene to benzoic acid
- Benzene is treated with alkyl halide in the presence of anhydrous lewis acid such as AlCl3, FeBr3. It gives alkyl benzene.
- Then alkyl benzene reacts with strong oxidizing agents with heating to give benzoic acid.
Questions
benzoic acid reacts with soda lime to give benzene. what is the reaction
When carboxylic acid is heated with soda lime (a mixture of calcium oxide and sodium hydroxide), -COOH part is removed and an alkane is given.
So, when benzoic acid is heated with soda lime to give benzene