Bromate ion (BrO3-) Ion Lewis Structure
Bromate ion contains one bromine atom and three oxygen atoms. Lewis structure of BrO3- contains two Br=O bonds and one Br-O bond. There is -1 charge on one oxygen atom in BrO3- lewis structure.
BrO3- lewis structure
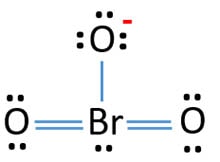
Oxygen atoms have made bonds with center bromine atom. From those bonds, there are two double bonds and one single bond in the BrO3- lewis structure. Also, there is one lone pair exist on bromine atom -1 charge exists on one oxygen atom in the lewis structure of BrO3- ion.
Steps of drawing lewis structure of BrO3- ion
When we draw a lewis structure, there are several guidelines to follow. Number of steps can be changed according the complexity of the molecule or ion. Because BrO3- ion is an ion and there are four atoms, we will have more steps than drawing a simple molecule or ion. However, those all steps are mentioned and explained in detail in this tutorial to improve your knowledge about lewis structure.
- Find total number of electrons of the valance shells of bromine atom and oxygen atoms
- Determine total electrons pairs existing as lone pairs and bonds
- Deciding center atom selection
- Mark lone pairs on atoms
- Mark charges on atoms if there are charges.
- Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure.
Total number of electrons of the valance shells of BrO3- ion
There are two elements in iodate ion; bromine and oxygen. Oxygen is located in group VIA in the periodic table and oxygen contains six electrons in its last shell (valence shell). Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of oxygen and bromine atoms.
- valence electrons given by oxygen atoms = 6 * 3 = 18
- valence electrons given by bromine atom = 7 * 1 = 7
- Due to -1 charge, one electron should be added to the total number of valence electrons.
- Total valence electrons = 18 + 7 + 1 = 26
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, BrO3- ion, Total pairs of electrons are thirteen in their valence shells.
Center atom of BrO3- molecule
Because, bromine can show higher valance than oxygen, bromine has the higher priority to be the center atom in BrO3-. Also, iodine is more electropositive than oxygen, it again proves, bromine should be the center atom.
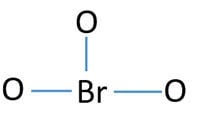
Lone pairs on atoms
After determining the center atom and sketch of BrO3- ion, we can start to mark lone pairs on atoms. Remember that, there are total of thirteen electron pairs.
- There are already three bonds in the drawn skeletal. So, now only ten electron pairs are remaining to mark as lone pairs.
- Usually, those remaining electron pairs should be started to mark on outside atoms. Therefore, we can start to mark those remaining electrons pairs on oxygen atoms. One oxygen atom will take three lone pairs. Then, nine electron pairs are marked on three oxygen atoms.
- Now, one electron pair is remaining and it is marked on bromine atom as following figure.
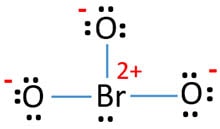
Mark charges on atoms
Each oxygen atom has -1 charge and bromine atom has +2 charge.
Check the stability and minimize charges on atoms by converting lone pairs to bonds
Because every atom has charges and having a charge like +2 ion iodine atom, above structure has to consider as not stable. Therefore, we should try to reduce charges by converting lone pairs to bonds to find the most stable structures.
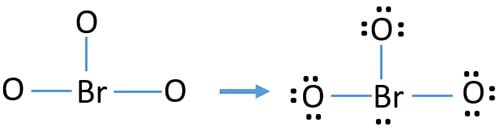
As below, we convert lone pairs of oxygen atoms to bonds in two steps.
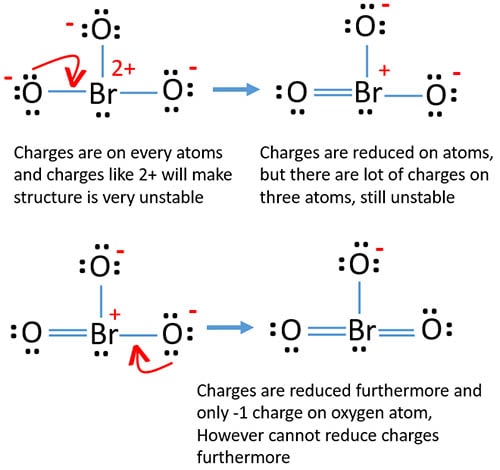
You can see, charges has been reduced in new structure than previous structures. There is only -1 charge on one oxygen atom.
Questions