Reaction of Benzene and Methyl Chloride with Anhydrous AlCl3
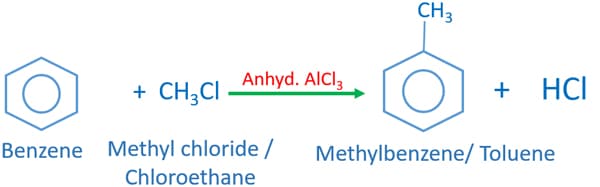
Toluene (methyl benzene) is given as the product when benzene reacts methyl chloride in presence of anhydrous Aluminium chloride (AlCl3). This reaction is defined as an electrophilic substitution reaction. AlCl3 catalysis the reaction and HCl molecules are formed as by-products during the reaction mechanism.
Content
- Preparation of methylbenzene by benzene
- Benzene + Methyl Chloride + Anhydrous AlCl3 → methylbenzene
- Catalyst behavior of anhydrous AlCl3
- Mechanism of benzene and methyl chloride reaction
- Toluene (Methylbenzene) reacts methyl chloride in presence of AlCl3
Preparation of methylbenzene by benzene
If excess methyl chloride is available, reaction continues. That means, given product by toluene reacts with more methyl chloride molecules and form more products. We will discuss those reactions later in this tutorial.
Friedel craft alkylation reaction
This is an example reaction to friedel craft alkylation. In a Friedel craft alkylation reaction, an alkyl chloride compound and a benzene or benzene substituted compound are involed in the presence of lewis acid catalyst.
Benzene + Methyl Chloride + Anhydrous AlCl3 → methylbenzene
C6H6 + CH3Cl + AlCl3 → C6HCH3 + HCl + AlCl3
Catalyst behavior of anhydrous AlCl3
Aluminium chloride is a lewis acid. As well, anhydrous aluminium chloride (AlCl3) is a catalyst and it increases the reaction rate. Therefore added AlCl3 do not change with reaction. As a by-product, HCl is given in the reaction.
Toluene has more names, methylbenzene, Phenylmethane. This reaction can be used to prepare benzoic acid as an initial step.
Mechanism of benzene and methyl chloride reaction
- As first step, +CH3 carbocation is generated. This cation is an electrophile and can attack parts which has higher electron density.
- +CH3 carbocation attacks the benzene ring and form an intermediate product as the below figure and that intermediate product also is a carbocation.
- This carbocation get a stability by sharing its positive charge through the benzene ring.
- Finally, hydrogen atom which is connected together with newly bonded -CH3 group is eliminated to give the desired final product.
Toluene (Methylbenzene) reacts methyl chloride in presence of AlCl3
Now, we are going to discuss, what will be happened if excess AlCl3 is there at the beginning of the reaction. First, toluene is prepared as described earlier.
Toluene reacts with more easily than benzene with methyl chloride in presence of AlCl3. This reaction will give two products 1,2-Dimethylbenzene and 1,4-Dimethylbenzene because toluene is a ortho-para activator.
Have Questions?
What is correct about following statements?
- For benzene alkylation, benzene reacts with methyl chloride.
- For benzene alkylation, benzene reacts with AlCl3.
- For benzene alkylation, benzene reacts with methyl chloride and anhydrous AlCl3.
- For benzene alkylation, benzene reacts with benzoyl chloride in presence of anhydrous AlCl3.
Statement 3 is correct.
In the statement 4, benzene reacts with benzoyl chloride in presence of anhydrous AlCl3. We call it acylation.
benzene react with benzoyl chloride in presence of anhydrous alcl3
Benzoyl chloride is an aromatic carboxylic acid chloride compound. As other aliphatic carboxylic acid chloride, benzoyl chloride reacts with benzene in presence of anhydrous AlCl3 and give Benzophenone (diphenylmethanone).
Why AlCl3 is used in the reaction of benzene and methyl chloride?
AlCl3 is a catalyst which reduces activation energy of the reaction of benzene and methyl chloride.
Will benzene + methyl chloride in presence of anhydrous AlCl3 give toxic compounds?
Reactants (benzene, methyl chloride) and given product methyl benzene are toxic compounds. Benzene was identified as a compound which can causes cancer in humans. When a man is exposed to benzene vapor, following signs and symptoms within minutes to several hours
- Drowsiness
- Dizziness
- Rapid or irregular heartbeat
- Headaches