Oxidation of Alcohols by PCC (Pyridinium chlorochromate)
PCC (Pyridinium chlorochromate) is a mild oxidizing agent used in organic chemistry. In this tutorial, we will study how different types of alcohols react differently with PCC and give products.
In this tutorial, we will discuss followings.
- Oxidation of primary, secondary alcohols by PCC
- Examples of primary alcohols oxidation to aldehyde
- Examples of secondary alcohols oxidation to aldehyde
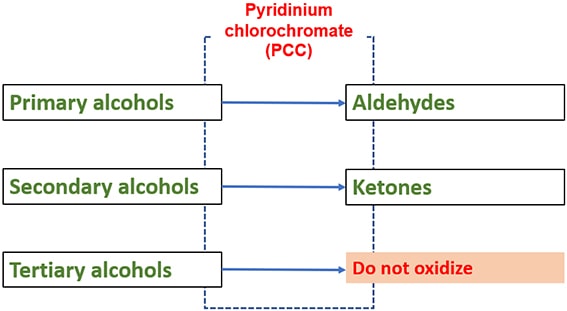
Primary alcohols, secondary alcohols and tertiary alcohols with PCC
Only primary and secondary alcohols are oxidized to aldehydes and ketones respectively. Tertiary alcohols are not oxidized by PCC.
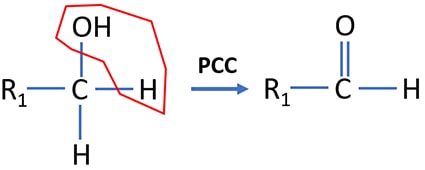
Primary alcohol oxidation by PCC
Primary alcohols are oxidized to aldehydes by PCC. During the process, a water molecule is eliminated from the alcohol molecule. If strong oxidizing agents such as acidic potassium permanganate is used as an oxidizing agent, primary alcohol is oxidized to a carboxylic acid.
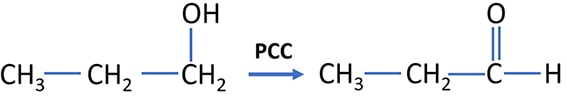
Oxidation of Ethanol by PCC reaction
Ethanol is oxidized to ethanal (acetaldehyde) by PCC. Oxidation state of the carbon atom (which is going to be oxidized), is changed from -1 to +1.
Propanol and PCC reaction
Propanol is oxidized to ethanoic acid (acetic acid) by PCC. Oxidation state of the carbon atom (which is going to be oxidized), is changed from -1 to +1.
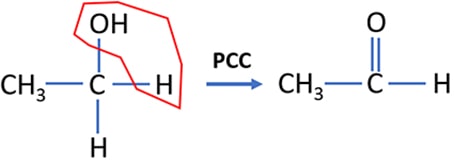
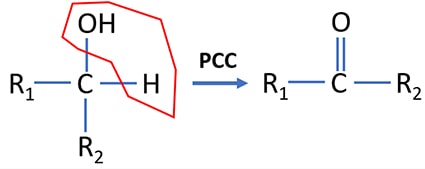
Secondary alcohol oxidation by PCC
Secondary alcohols are oxidized to ketones by PCC. During the process, a water molecule is eliminated from the secondary alcohol molecule. If strong oxidizing agents such as acidic potassium permanganate is used as an oxidizing agent, primary alcohol is oxidized to a ketone.
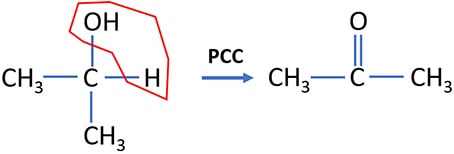
2-propanol and PCC reaction
2-propanol is oxidized to propanone by PCC. Oxidation state of the carbon atom (which is going to be oxidized), is changed from 0 to +2.
Why are tertiary alcohols not oxidized by PCC?
There are three C-C bonds around the carbon atom which has bonded with the -OH group. Therefore, there is no Hydrogen atom which has a joint with that carbon atom. Breaking those three C-C bonds is not easy because it needs a lot of energy. As PCC, strong oxidizing agents also cannot oxidize tertiary alcohols.
Questions
Is PCC a strong oxidizing agent
PCC is not a strong oxidizing agent. PCC is a mild oxidizing agent.
what does pcc do in a reaction
PCC is a mild oxidizing agent and used to oxidize primary and secondary alcohols to ketones or aldehydes.